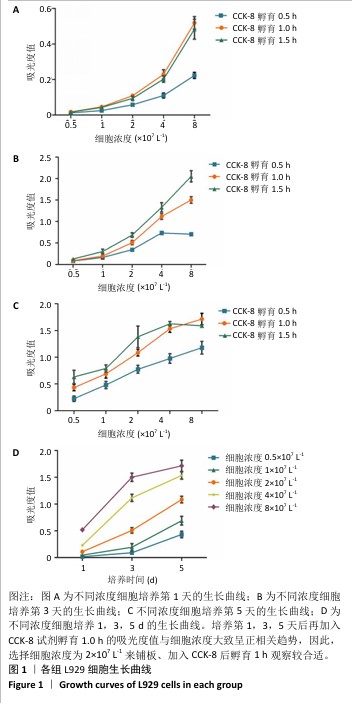
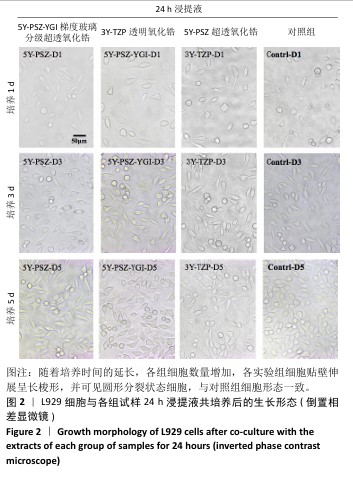
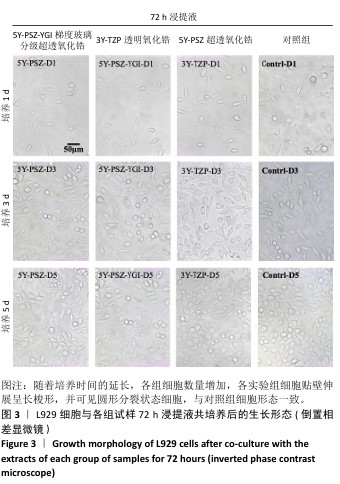
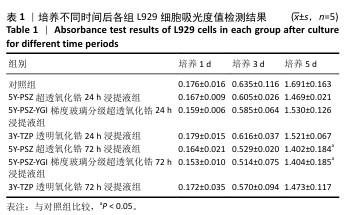
[1] SAHOO N, CARVALHO O, ÖZCAN M, et al. Ultrashort pulse laser patterning of zirconia (3Y-TZP) for enhanced adhesion to resin-matrix cements used in dentistry: An integrative review. J Mech Behav Biomed Mater. 2023;143:105943.
[2] DE AZEVEDO-SILVA LJ, FERRAIRO BM, MINIM PR, et al. Bovine hydroxyapatite/3Y-TZP bioceramic: Aligning 3Y-TZP content with sintering parameters. J Mech Behav Biomed Mater. 2024;156: 106569.
[3] BALTAZAR J, RODRIGUES PAIS ALVES MF, MARTINS MA, et al. Flexural strength of 3Y-TZP bioceramics obtained by direct write assembly as function of residual connected-porosity. J Mech Behav Biomed Mater. 2022;126:105035.
[4] RUTKUNAS V, BORUSEVICIUS R, BALCIUNAS E, et al. The Effect of UV Treatment on Surface Contact Angle, Fibroblast Cytotoxicity, and Proliferation with Two Types of Zirconia-Based Ceramics. Int J Environ Res Public Health. 2022;19(17):11113.
[5] RODRÍGUEZ-LOZANO FJ, LÓPEZ-GARCÍA S, SÁNCHEZ-BAUTISTA S, et al. Effect of milled and lithography-based additively manufactured zirconia (3Y-TZP) on the biological properties of human osteoblasts. J Prosthet Dent. 2023;130(6):889-896.
[6] SURANA P, SINGH DHULL K, ARYA A, et al. Bio-ceramics application in Dentistry. Bioinformation. 2024;20(2):136-139.
[7] OH JW, SONG KY, AHN SG, et al. Effects of core characters and veneering technique on biaxial flexural strength in porcelain fused to metal and porcelain veneered zirconia. J Adv Prosthodont. 2015; 7(5):349-357.
[8] ZHU DB, LIANG JP, QU YX, et al. Functionalized bio-artifact fabricated via selective slurry extrusion. Part 2: Fabrication of ceramic dental crown. J Nanosci Nanotechnol. 2014;14(5): 3703-3706.
[9] SAILER I, STRASDING M, VALENTE NA, et al. A systematic review of the survival and complication rates of zirconia-ceramic and metal-ceramic multiple-unit fixed dental prostheses. Clin Oral Implants Res. 2018; 29 Suppl 16:184-198.
[10] AKASHI Y, SHIMOO Y, HASHIGUCHI H, et al. Effects of Excimer Laser Treatment of Zirconia Disks on the Adhesion of L929 Fibroblasts. Materials (Basel). 2022;16(1):115.
[11] HU J, QIE Y, LUO Y, et al. Effect of Porous Zirconia Coating on Human Gingival Fibroblasts and Its Mechanism. J Biomed Nanotechnol. 2022;18(4):1164-1171.
[12] YANG Z, LIU M, YANG Y, et al. Biofunctionalization of zirconia with cell-adhesion peptides via polydopamine crosslinking for soft tissue engineering: effects on the biological behaviors of human gingival fibroblasts and oral bacteria. RSC Adv. 2020;10(11):6200-6212.
[13] JERMAN E, LÜMKEMANN N, EICHBERGER M, et al. Evaluation of translucency, Marten’s hardness, biaxial flexural strength and fracture toughness of 3Y-TZP, 4Y-TZP and 5Y-TZP materials. Dent Mater. 2021; 37(2):212-222.
[14] KIM JW, VO TV, SATPATHY M, et al. Improving Fracture Resistance of 5Y-PSZ-based Three-unit Bridge Prosthesis. Int J Prosthodont. 2023. doi: 10.11607/ijp.8643.
[15] INOKOSHI M, SHIMIZU H, NOZAKI K, et al. Crystallographic and morphological analysis of sandblasted highly translucent dental zirconia. Dent Mater. 2018;34(3):508-518.
[16] DE ARAÚJO-JÚNIOR ENS, BERGAMO ETP, BASTOS TMC, et al. Ultra-translucent zirconia processing and aging effect on microstructural, optical, and mechanical properties. Dent Mater. 2022;38(4): 587-600.
[17] MAO L, KAIZER MR, ZHAO M, et al. Graded Ultra-Translucent Zirconia (5Y-PSZ) for Strength and Functionalities. J Dent Res. 2018;97(11): 1222-1228.
[18] ELSHAHAWY WM, WATANABE I, KRAMER P. In vitro cytotoxicity evaluation of elemental ions released from different prosthodontic materials. Dent Mater. 2009;25(12):1551-1555.
[19] YANG R, AROLA D, HAN Z, et al. A comparison of the fracture resistance of three machinable ceramics after thermal and mechanical fatigue. J Prosthet Dent. 2014;112(4):878-885.
[20] ARCILA LVC, RAMOS NC, CAMPOS TMB, et al. Mechanical behavior and microstructural characterization of different zirconia polycrystals in different thicknesses. J Adv Prosthodont. 2021;13(6):385-395.
[21] COKIC SM, VLEUGELS J, VAN MEERBEEK B, et al. Mechanical properties, aging stability and translucency of speed-sintered zirconia for chairside restorations. Dent Mater. 2020;36(7): 959-972.
[22] MIURA S, SHINYA A, KOIZUMI H, et al. Effect of speed sintering of monolithic zirconia with different yttria contents on color and crystal phase. Eur J Oral Sci. 2022;130(6):e12898.
[23] MÜLLER B P, ENSSLEN S, DOTT W, et al. Improved sample preparation of biomaterials for in vitro genotoxicity testing using reference materials . J Biomed Mater Res. 2002;61(1):83-90.
[24] LEMONS JE. Ceramics: past, present, and future. Bone. 1996;19(1 Suppl): 121s-128s.
[25] CRAWFORD L, WYATT M, BRYERS J, et al. Biocompatibility Evolves: Phenomenology to Toxicology to Regeneration. Adv Healthc Mater. 2021;10(11):e2002153.
[26] JURAK M, WIĄCEK AE, ŁADNIAK A, et al. What affects the biocompatibility of polymers? Adv Colloid Interface Sci. 2021;294: 102451.
[27] HAO Y, HUANG X, ZHOU X, et al. Influence of Dental Prosthesis and Restorative Materials Interface on Oral Biofilms. Int J Mol Sci. 2018; 19(10):3157.
[28] MITTERMÜLLER P, HILLER KA, SCHMALZ G, et al. Five hundred patients reporting on adverse effects from dental materials: Frequencies, complaints, symptoms, allergies. Dent Mater. 2018; 34(12):1756-1768.
[29] WELLER J, VASUDEVAN P, KREIKEMEYER B, et al. The role of bacterial corrosion on recolonization of titanium implant surfaces: An in vitro study. Clin Implant Dent Relat Res. 2022;24(5):664-675.
[30] 殷隽雅.新型齿科可切削复合树脂陶瓷的细胞生物相容性初探[D].北京:首都医科大学,2017.
[31] SRIMANEEPONG V, HEBOYAN A, ZAFAR MS, et al. Fixed Prosthetic Restorations and Periodontal Health: A Narrative Review. J Funct Biomater. 2022;13(1):15.
[32] RINGEISEN H, PÖSCHKE A, KRÄHLING B, et al. Influence of dental materials on cells of the equine periodontium. Equine Vet J. 2018; 50(3):363-369.
[33] ELSHAHAWY W, WATANABE I, KOIKE M. Elemental ion release from four different fixed prosthodontic materials. Dent Mater. 2009;25(8):
976-981.
[34] PARK YJ, SONG YH, AN JH, et al. Cytocompatibility of pure metals and experimental binary titanium alloys for implant materials. J Dent. 2013;41(12):1251-1258.
[35] MILLEDING P, HARALDSSON C, KARLSSON S. Ion leaching from dental ceramics during static in vitro corrosion testing. J Biomed Mater Res. 2002;61(4):541-550.
[36] LEINFELDER KF. Ask the expert. Will ceramic restorations be challenged in the future? J Am Dent Assoc. 2001;132(1):46-47.
[37] GALANTE R, FIGUEIREDO-PINA CG, SERRO AP. Additive manufacturing of ceramics for dental applications: A review. Dent Mater. 2019;35(6): 825-846.
[38] MALOO LM, PATEL A, TOSHNIWAL SH, et al. Smart Materials Leading to Restorative Dentistry: An Overview. Cureus. 2022; 14(10):e30789.
[39] Li K, Yao C, Sun Y, et al. Enhancing resin-dentin bond durability using a novel mussel-inspired monomer. Mater Today Bio. 2021; 12:100174.
[40] ATAY A, GÜRDAL I, BOZOK ÇETıNTAS V, et al. Effects of New Generation All-Ceramic and Provisional Materials on Fibroblast Cells. J Prosthodont. 2019;28(1):e383-e394.
[41] GUESS WL, ROSENBLUTH SA, SCHMIDT B, et al. Agar diffusion method for toxicity screening of plastics on cultured cell monolayers. J Pharm Sci. 1965;54(10):1545-1547.
[42] DUZYOL M, BAYRAM P, DUZYOL E, et al. Assessing the impact of dental restorative materials on fibroblast cells: an immunohistochemical and ELISA analysis. Sci Rep. 2024;14(1):4725.
[43] QU Y, KANG M, DONG R, et al. Evaluation of a new Mg-Zn-Ca-Y alloy for biomedical application. J Mater Sci Mater Med. 2015; 26(1):5342.
[44] MALABANAN JWT, ALCANTARA KP, JANTARATANA P, et al. Enhancing Physicochemical Properties and Biocompatibility of Hollow Porous Iron Oxide Nanoparticles through Polymer-Based Surface Modifications. ACS Appl Bio Mater. 2023;6(12):5426-5441.
[45] 翟建才,赵南明.生物材料与血液界面作用机制及相容性评价的研究技术[J].国外医学生物医学工程分册,1995,18(1):6-12.
[46] BRAUNE S, LATOUR RA, REINTHALER M, et al. In Vitro Thrombogenicity Testing of Biomaterials. Adv Healthc Mater. 2019;8(21):e1900527. |