中国组织工程研究 ›› 2024, Vol. 28 ›› Issue (34): 5467-5472.doi: 10.12307/2024.832
• 软骨组织构建 cartilage tissue construction • 上一篇 下一篇
地黄梓醇调控ATDC5软骨细胞的衰老
贾瑞英1,梅 杰2,何 强2,李 丹1,孙 欣3,钱卫庆3,刘 振1
- 1菏泽市立医院,山东省菏泽市 274000;2山东中医药大学附属医院,山东省济南市 250011;3南京中医药大学附属南京中医院骨伤科,江苏省南京市 210022
Catalpol from Rehmannia glutinosa regulates senescence in ATDC5 chondrocytes
Jia Ruiying1, Mei Jie2, He Qiang2, Li Dan1, Sun Xin3, Qian Weiqing3, Liu Zhen1
- 1Heze Municipal Hospital, Heze 274000, Shandong Province, China; 2Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan 250011, Shandong Province, China; 3Department of Orthopedics and Traumatology, Nanjing Hospital of Traditional Chinese Medicine Affiliated to Nanjing University of Chinese Medicine, Nanjing 210022, Jiangsu Province, China
摘要:
文题释义:
地黄梓醇:熟地黄是中国“四大怀药”之一,记载可追溯至《神农本草经》。地黄是玄参科地黄的新鲜或干燥块根,梓醇是一种从熟地黄中分离得到的环烯醚萜葡萄糖苷,是熟地黄的主要活性成分,药理作用包括抗炎、抗氧化等作用。细胞衰老:作为生物体所必然经历的自然过程,在整个生命周期中均可能发生,其突出的特点表现为细胞永久性的处于增殖停滞的状态,而衰老细胞的持续性存在和逐渐积累会分泌出大量的促炎性蛋白,进而会严重损害机体组织的功能。
背景:课题组前期体内、体外研究结果表明地黄梓醇能够显著降低膝骨关节炎大鼠滑膜组织中炎症指标水平,同时能够延缓膝骨关节炎进展,但是否通过影响软骨细胞衰老进而延缓膝骨关节炎的进展尚未明确。
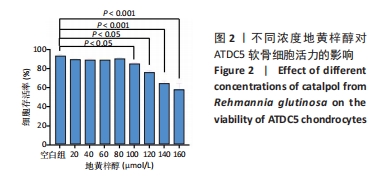
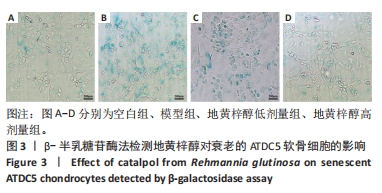
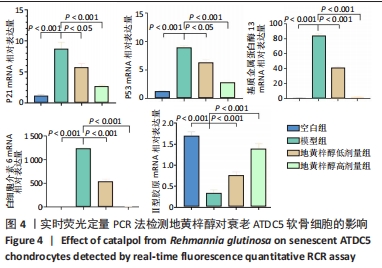
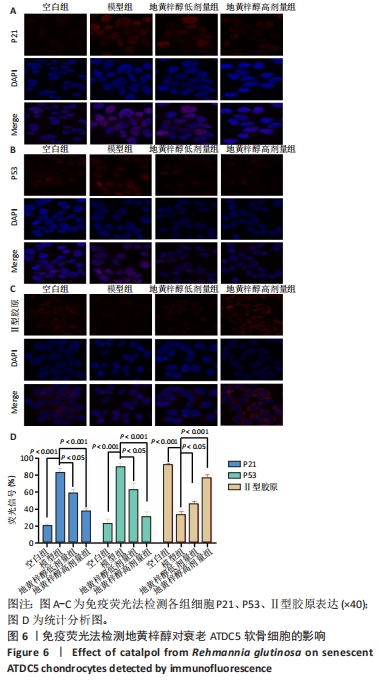
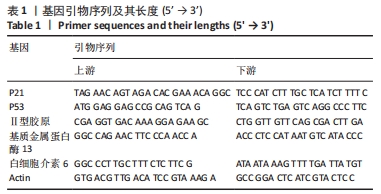
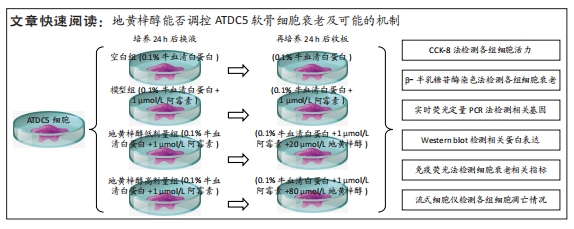
目的:探讨地黄梓醇能否调控ATDC5软骨细胞衰老及可能的机制。方法:将ATDC5软骨细胞分为空白组(0.1%牛血清白蛋白)、模型组(0.1%牛血清白蛋白 +1 µmol/L阿霉素)、地黄梓醇低剂量组(0.1%牛血清白蛋白+1 µmol/L阿霉素+20 µmol/L地黄梓醇)及地黄梓醇高剂量组(0.1%牛血清白蛋白+1 µmol/L阿霉素+80 µmol/L地黄梓醇)。应用阿霉素诱导构建ATDC5软骨细胞衰老模型,按上述分组予以对应的处理。cck-8法检测地黄梓醇对ATDC5软骨细胞活力的影响,筛选地黄梓醇最佳给药浓度。相应处理后应用β-半乳糖苷酶染色法检测各组ATDC5软骨细胞衰老情况;实时荧光定量PCR法检测相关基因表达(P21、P53、Ⅱ型胶原、基质金属蛋白酶13、白细胞介素6);Western blot检测P21、P53、Ⅱ型胶原、基质金属蛋白酶13、白细胞介素6的表达水平;免疫荧光法检测P21、P53和Ⅱ型胶原表达情况;流式细胞仪检测各组细胞凋亡情况。
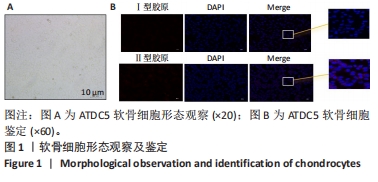
结果与结论:①经鉴定成功诱导ATDC5软骨细胞并诱导衰老模型;②地黄梓醇浓度在0,20,40,80 µmol/L时对细胞活力均无明显影响,提示地黄梓醇对细胞无毒性,可安全使用(P > 0.05);当浓度≥100 µmol/L时,细胞活力降低,提示可能存在毒性,故选择80 µmol/L作为高剂量进行后续实验;③与空白组β-半乳糖苷酶阳性细胞百分率(17.32±0.72)%比较,模型组(86.93±2.18)%显著升高(P < 0.05);与模型组比较,地黄梓醇低、高剂量组(57.28±1.73)%、(27.18±0.97)%均显著降低(P < 0.05);④与模型组比较,地黄梓醇低、高剂量组的P21、P53、基质金属蛋白酶13、白细胞介素6 mRNA和蛋白相对表达量均显著下调,而Ⅱ型胶原的mRNA和蛋白相对表达量显著上调(P < 0.05),高剂量组更为明显(P < 0.05);⑤与模型组比较,地黄梓醇低、高剂量组的P21、P53荧光信号均显著减弱,而Ⅱ型胶原的荧光信号显著增强(P < 0.05),高剂量组更为明显(P < 0.05);⑥经Annexin V/PI法检测各组细胞凋亡情况,与空白组比较,模型组的凋亡情况无明显变化(P > 0.05);与模型组比较,地黄梓醇低、高剂量组的凋亡指标均显著升高,且以高剂量组更为明显(P < 0.05);⑦提示地黄梓醇能够通过促进衰老的ATDC5软骨细胞凋亡,进一步清除衰老的ATDC5软骨细胞,降低衰老相关表型进而延缓骨关节炎的进展。
https://orcid.org/0009-0008-9021-8156(贾瑞英)
中国组织工程研究杂志出版内容重点:组织构建;骨细胞;软骨细胞;细胞培养;成纤维细胞;血管内皮细胞;骨质疏松;组织工程
中图分类号: