[1] LI X, YIN Z, LI X, et al. Efficacy of moxibustion for primary osteoporosis: a trial sequential meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. 2022;2022:1268876.
[2] GAO Y, PATIL S, JIA J. The development of molecular biology of osteoporosis. Int J Mol Sci. 2021;22(15):8182.
[3] 柯呈辉,何立江,吴文华.双膦酸盐防治骨质疏松性骨折的研究进展[J].中国骨质疏松杂志,2019,25(6):870-874.
[4] 舒晓春,刘君静,朱丹华,等.不同浓度的骨碎补总黄酮对大鼠骨髓间充质干细胞向成骨细胞分化的影响[J].中国病理生理杂志,2010,26(7): 1261-1264.
[5] 邓强,乔小万,李中锋,等.骨碎补活性成分治疗骨骼系统疾病研究进展[J].辽宁中医药大学学报,2022,24(7):1-5.
[6] WANG Y, WU H, CHEN P, et al. Fertility and early embryonic development toxicity assessment of naringin in Sprague-Dawley rats. Regul Toxicol Pharmacol. 2021;123:104938.
[7] AN J, YANG H, ZHANG Q, et al. Natural products for treatment of osteoporosis: The effects and mechanisms on promoting osteoblast-mediated bone formation. Life Sci. 2016;147:46-58.
[8] ALAM MA, SUBHAN N, RAHMAN MM, et al. Effect of citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms of action. Adv Nutr. 2014;5(4):404-417.
[9] RAJA KUMAR S, MOHD RAMLI ES, Abdul Nasir NA, et al. Preventive effect of naringin on metabolic syndrome and its mechanism of action: a systematic review. Evid Based Complement Alternat Med. 2019;2019:9752826.
[10] FREEDMAN L, MERRITT AJ. Citrus flavonoid complex: chemical fractionation and biological activity. Science. 1963;139(3552):344-345.
[11] KROYER G. Uber die antioxidative Aktivität von Zitrusfruchtschalen [The antioxidant activity of citrus fruit peels]. Z Ernahrungswiss. 1986;25(1):63-69.
[12] 史万忠,徐德生,沈培芝,等.补肾益精方水提物和醇提物化学指标及药效的比较研究[J].中国中药杂志,2001,26(7):28-31.
[13] ZHANG P, DAI KR, YAN SG, et al. Effects of naringin on the proliferation and osteogenic differentiation of human bone mesenchymal stem cell. Eur J Pharmacol. 2009;607(1-3):1-5.
[14] ANG ES, YANG X, CHEN H, et al. Naringin abrogates osteoclastogenesis and bone resorption via the inhibition of RANKL-induced NF-κB and ERK activation. FEBS Lett. 2011;585(17):2755-2762.
[15] CAO X, LIN W, LIANG C, et al. Naringin rescued the TNF-α-induced inhibition of osteogenesis of bone marrow-derived mesenchymal stem cells by depressing the activation of NF-кB signaling pathway. Immunol Res. 2015; 62(3):357-367.
[16] MA X, LV J, SUN X, et al. Naringin ameliorates bone loss induced by sciatic neurectomy and increases Semaphorin 3A expression in denervated bone. Sci Rep. 2016;6:24562.
[17] MARTINIAKOVA M, BABIKOVA M, MONDOCKOVA V, et al. The role of macronutrients, micronutrients and flavonoid polyphenols in the prevention and treatment of osteoporosis. Nutrients. 2022;14(3):523.
[18] 上官文姬,张跃辉,岳江,等.柚皮苷通过HIF-1α/VEGF信号促进H型血管抗骨质疏松的研究[J].中国骨质疏松杂志,2022,28(12):1755-1759.
[19] RIVOIRA M A, RIGALLI A, CORBALL L, et al. Naringin prevents bone damage in the experimental metabolic syndrome induced by a fructose-rich diet. Appl Physiol Nutr Metab. 2022;47(4):395-404.
[20] WANG W, MAO J, CHEN Y, et al. Naringin promotes osteogenesis and ameliorates osteoporosis development by targeting JAK2/STAT3 signalling. Clin Exp Pharmacol Physiol. 2022;49(1):113-121.
[21] PANG WY, WANG XL, MOK SK, et al. Naringin improves bone properties in ovariectomized mice and exerts oestrogen-like activities in rat osteoblast-like (UMR-106) cells. Br J Pharmacol. 2010;159(8):1693-1703.
[22] JIN H, JIANG N, XU W, et al. Effect of flavonoids from Rhizoma Drynariae on osteoporosis rats and osteocytes. Biomed Pharmacother. 2022;153:113379.
[23] LI Y, LIU J, ZHOU H, et al. Liquid chromatography-mass spectrometry method for discovering the metabolic markers to reveal the potential therapeutic effects of naringin on osteoporosis. J Chromatogr B Analyt Technol Biomed Life Sci. 2022;1194:123170.
[24] LI XL, XU F, LIN FH, et al. A naringin- and icariin-contained herbal formula, gushukang, ameliorated aged osteoporosis of aged mice with high calcium intake. Am J Chin Med. 2020;48(7):1671-1691.
[25] LI C, ZHANG J, LV F, et al. Naringin protects against bone loss in steroid-treated inflammatory bowel disease in a rat model. Arch Biochem Biophys. 2018;650:22-29.
[26] 刘小坡,冯云波,曹国龙,等.柚皮苷对老年大鼠骨质疏松性骨折愈合的影响[J].中国临床药理学杂志,2020,36(9):1117-1120.
[27] SONG N, ZHAO Z, MA X, et al. Naringin promotes fracture healing through stimulation of angiogenesis by regulating the VEGF/VEGFR-2 signaling pathway in osteoporotic rats. Chem Biol Interact. 2017;261:11-17.
[28] LI N, JIANG Y, WOOLEY PH, et al. Naringin promotes osteoblast differentiation and effectively reverses ovariectomy-associated osteoporosis. J Orthop Sci. 2013;18(3):478-485.
[29] 袁毅,傅裕,许东,等.柚皮苷联合脉冲电磁场对大鼠股骨骨折愈合的影响[J].中国矫形外科杂志,2018,26(6):543-547.
[30] 卢育南,张信照,林斌斌,等.柚皮苷-壳聚糖/羟基磷灰石复合支架修复大鼠颅骨缺损[J].中国组织工程研究,2022,26(28):4441-4445.
[31] GE X, ZHOU G. Protective effects of naringin on glucocorticoid-induced osteoporosis through regulating the PI3K/Akt/mTOR signaling pathway. Am J Transl Res. 2021;13(6):6330-6341.
[32] ZHU Z, WANG Z, MA C, et al. Isopsoralen promotes osteogenic differentiation of human jawbone marrow mesenchymal cells through Notch signaling pathway. Ann Anat. 2023;250:152156.
[33] GUO J, TONG CY, SHI JG, et al. Deletion of osteopontin in non-small cell lung cancer cells affects bone metabolism by regulating miR-34c/Notch1 axis: a clue to bone metastasis. Eur J Histochem. 2023;67(3):3631.
[34] ZHANG W, BAI J, LI L, et al. EGFL7 secreted by human bone mesenchymal stem cells promotes osteoblast differentiation partly via downregulation of Notch1-Hes1 signaling pathway. Stem Cell Rev Rep. 2023;19(4):968-982.
[35] ZHAO ZH, MA XL, MA JX, et al. Sustained release of naringin from silk-fibroin-nanohydroxyapatite scaffold for the enhancement of bone regeneration. Mater Today Bio. 2022;13:100206.
[36] XU M, SONG D, XIE X, et al. CGK733 alleviates ovariectomy-induced bone loss through blocking RANKL-mediated Ca2+ oscillations and NF-κB/MAPK signaling pathways. iScience. 2023;26(10):107760.
[37] HU R, CHRN L, CHEN X, et al. Aloperine improves osteoporosis in ovariectomized mice by inhibiting RANKL-induced NF-κB, ERK and JNK approaches. Int Immunopharmacol. 2021;97:107720.
[38] LIU CL, HO TL, FANG SY, et al, Tang CH. Ugonin L inhibits osteoclast formation and promotes osteoclast apoptosis by inhibiting the MAPK and NF-κB pathways. Biomed Pharmacother. 2023;166:115392.
[39] YU X, WU Q, REN Z, et al. Kaempferol attenuates wear particle-induced inflammatory osteolysis via JNK and p38-MAPK signaling pathways. J Ethnopharmacol. 2024;318(Pt B):117019.
[40] 汪甜,杨丽,张荣华.柚皮苷在促大鼠骨髓间充质干细胞骨向分化过程中对MAPK信号通路的影响[J].中国病理生理杂志,2012,28(5):769-776.
[41] FAN J, ZHANG X, KANG M, et al. Complementary modulation of BMP signaling improves bone healing efficiency. Biomaterials. 2023;302:122335.
[42] ZENG L, GU R, LI W, et al. Ataluren prevented bone loss induced by ovariectomy and aging in mice through the BMP-SMAD signaling pathway. Biomed Pharmacother. 2023;166:115332.
[43] 宁宇,刘想忠,汪伟,等.柚皮苷通过Runx2信号通路促进MSCs成骨分化的实验研究[J].湖北中医药大学学报,2019,21(1):9-14.
[44] LI X, LIU C, ZHANG X, et al. Bruceine A: suppressing metastasis via MEK/ERK pathway and invoking mitochondrial apoptosis in triple-negative breast cancer. Biomed Pharmacother. 2023;168:115784.
[45] MA C, MO L, WANG Z, et al. Dihydrotanshinone I attenuates estrogen-deficiency bone loss through RANKL-stimulated NF-κB, ERK and NFATc1 signaling pathways. Int Immunopharmacol. 2023;123:110572.
[46] ZHANG C, LIU M, WANG X, et al. ALP inhibitors inhibit inflammatory responses and osteoblast differentiation in hVIC via AKT-ERK pathways. Altern Ther Health Med. 2023;29(1):58-65.
[47] 周森,孙奇峰,蒋雷,等.失重下柚皮苷作用于T细胞干预的成骨细胞增殖分化研究[J].中国骨质疏松杂志,2022,28(8):1099-1103.
[48] LIU Y, ZHAO L, HE X, et al. Jintiange proteins promote osteogenesis and inhibit apoptosis of osteoblasts by enhancing autophagy via PI3K/AKT and ER stress pathways. J Ethnopharmacol. 2023;311:116399.
[49] MA Y, HU J, SONG C, et al. Er-Xian decoction attenuates ovariectomy-induced osteoporosis by modulating fatty acid metabolism and IGF1/PI3K/AKT signaling pathway. J Ethnopharmacol. 2023;301:115835.
[50] XU X, WANG H, LU X, et al. Sodium p-hydroxybenzoate alleviates osteoporosis through inhibiting bone metabolism and oxidative stress via activating ERα. Pak J Pharm Sci. 2023;36(5):1415-1424.
[51] CHEN G, CHEN Y, HONG J, et al. Secoisolariciresinol diglucoside regulates estrogen receptor expression to ameliorate OVX-induced osteoporosis. J Orthop Surg Res. 2023;18(1):792.
[52] WU GJ, CHEN KY, YANG JD, et al . Naringin improves osteoblast mineralization and bone healing and strength through regulating estrogen receptor alpha-dependent alkaline phosphatase gene expression. J Agric Food Chem. 2021;69(44):13020-13033.
[53] LI S, CUI Y, LI M, et al. Acteoside derived from cistanche improves glucocorticoid-induced osteoporosis by activating PI3K/AKT/mTOR pathway. J Invest Surg. 2023;36(1):2154578.
[54] WANG Y, GAoO Y, WANG Y, et al, He H. GDNF promotes the proliferation and osteogenic differentiation of jaw bone marrow mesenchymal stem cells via the Nr4a1/PI3K/Akt pathway. Cell Signal. 2023;108:110721.
[55] SHANG J, YU Z, XIONG C, et al. Resistin targets TAZ to promote osteogenic differentiation through PI3K/AKT/mTOR pathway. iScience. 2023;26(7): 107025.
[56] 林春淑,舒晓春,肖菲娜,等.柚皮苷对高糖作用下MC3T3-E1细胞活力和Akt通路相关因子表达的影响[J].中华细胞与干细胞杂志(电子版), 2020,10(6):321-327.
[57] 容婵,廖莉娅,林道建,等.柚皮苷对地塞米松诱导的小鼠MC3T3-E1细胞凋亡及线粒体凋亡途径的影响[J].临床和实验医学杂志,2017, 16(5):417-420.
[58] LIN BH, MA RX, WU JT, et al. Cinnamaldehyde alleviates bone loss by targeting oxidative stress and mitochondrial damage via the Nrf2/HO-1 pathway in BMSCs and ovariectomized mice. J Agric Food Chem. 2023. doi: 10.1021/acs.jafc.3c03501.
[59] FAN JB, YUAN K, ZHU XH,et al. Neuroligin-3 activates Akt-dependent Nrf2 cascade to protect osteoblasts from oxidative stress. Free Radic Biol Med. 2023;208:807-819.
[60] WANG L, ZHANG YG, WANG XM, et al. Naringin protects human adipose-derived mesenchymal stem cells against hydrogen peroxide-induced inhibition of osteogenic differentiation. Chem Biol Interact. 2015;242: 255-261.
[61] WANG F, YANG G, LI Y, et al. A peptide from wheat germ abolishes the senile osteoporosis by regulating OPG/RANKL/RANK/TRAF6 signaling pathway. Phytomedicine. 2022;104:154304.
[62] HASANI S, FATHABADI F, SAEIDI S, et al. The role of NFATc1 in the progression and metastasis of prostate cancer: A review on the molecular mechanisms and signaling pathways. Cell Biol Int. 2023;47(12):1895-1904.
[63] XU H, JIA Y, LI J, et al. Niloticin inhibits osteoclastogenesis by blocking RANKL-RANK interaction and suppressing the AKT, MAPK, and NF-κB signaling pathways. Biomed Pharmacother. 2022;149:112902.
[64] HUANG X, LI Y, LIAO H, et al. Research advances on stem cell-derived extracellular vesicles promoting the reconstruction of alveolar bone through RANKL/RANK/OPG pathway. J Funct Biomater. 2023;14(4):193.
[65] 李风波,孙晓雷,马剑雄,等.柚皮苷对破骨细胞凋亡的影响[J].中国矫形外科杂志,2021,29(5):450-454.
[66] 李风波,孙晓雷,马剑雄,等.柚皮苷对破骨细胞分化的影响[J].中国中药杂志,2015,40(2):308-312.
[67] YANG C, LIU W, SHAN H, et al.Naringin inhibits titanium particles-induced up-regulation of TNF-α and IL-6 via the p38 MAPK pathway in fibroblasts from hip periprosthetic membrane. Connect Tissue Res. 2021;62(5): 485-494.
[68] XU T, WANG L, TAO Y, et al. The function of naringin in inducing secretion of osteoprotegerin and inhibiting formation of osteoclasts. Evid Based Complement Alternat Med. 2016;2016:8981650.
[69] 刘朝晖,马剑雄,马信龙.骨质疏松症的治疗及柚皮苷抗骨质疏松的研究进展[J].中国骨质疏松杂志,2020,26(4):615-618,624.
|
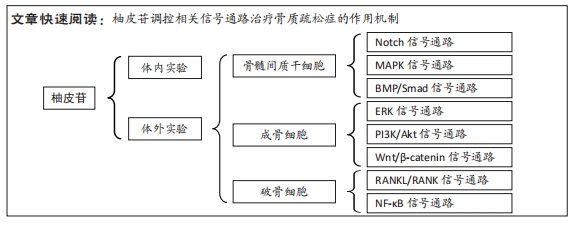
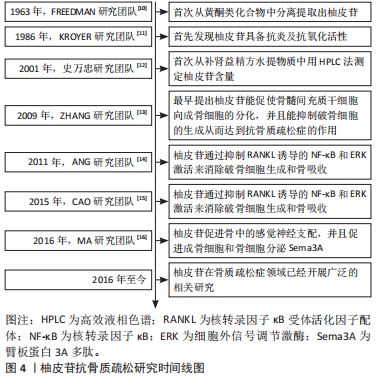
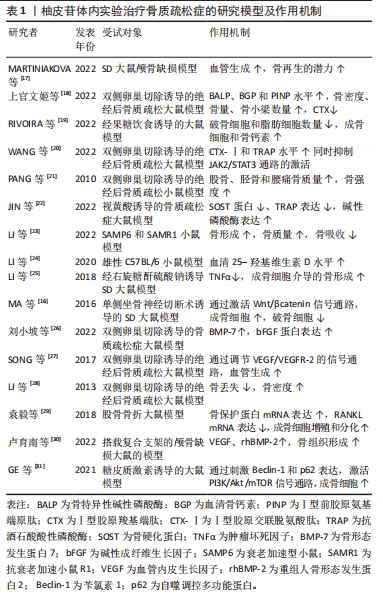

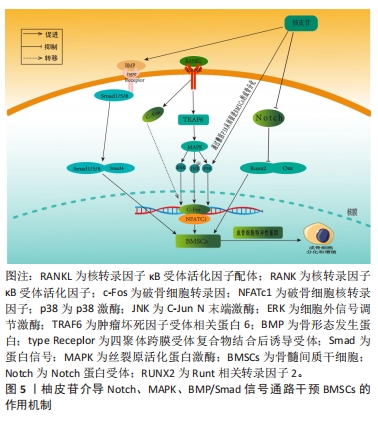
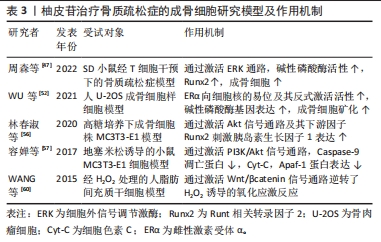
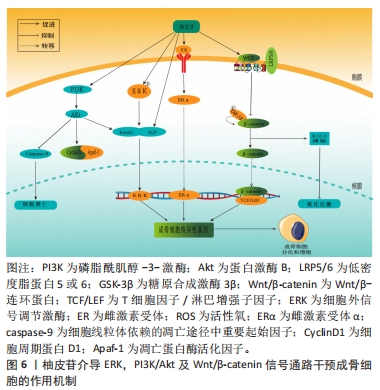
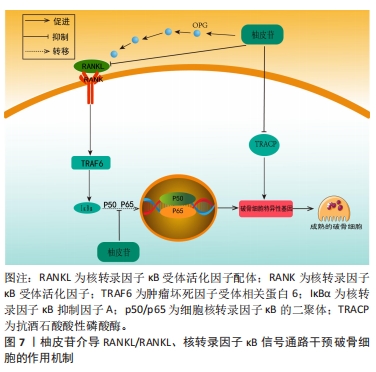
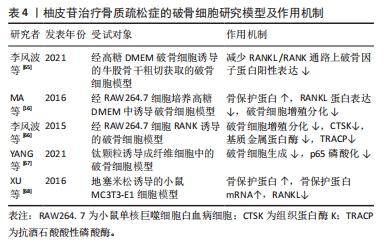
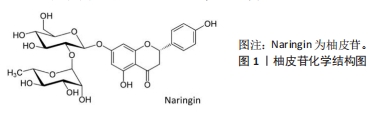 随着骨组织工程技术的发展,利用柚皮苷与羟基磷灰石纳米颗粒的复合水凝胶支架来治疗骨质疏松症已经成为近期的研究热点,并展现优良的应用潜力。因此,柚皮苷对骨质疏松症的治疗具有多元性且兼具经济性,若明确柚皮苷对该病相关信号通路的影响,可进一步提高对柚皮苷治疗骨质疏松症相关机制的认识。此前已有针对中药治疗骨质疏松症的相关机制研究的综述,但其研究范围较为广泛,对柚皮苷干预该病的具体机制论述深度不足。
随着骨组织工程技术的发展,利用柚皮苷与羟基磷灰石纳米颗粒的复合水凝胶支架来治疗骨质疏松症已经成为近期的研究热点,并展现优良的应用潜力。因此,柚皮苷对骨质疏松症的治疗具有多元性且兼具经济性,若明确柚皮苷对该病相关信号通路的影响,可进一步提高对柚皮苷治疗骨质疏松症相关机制的认识。此前已有针对中药治疗骨质疏松症的相关机制研究的综述,但其研究范围较为广泛,对柚皮苷干预该病的具体机制论述深度不足。