中国组织工程研究 ›› 2024, Vol. 28 ›› Issue (31): 5022-5028.doi: 10.12307/2024.726
• 干细胞基础实验 basic experiments of stem cells • 上一篇 下一篇
黄芪甲苷可抑制炎性诱导星形胶质细胞激活及炎症反应
于婧文1,郭敏芳1,张冰心1,穆秉桃1,孟 涛1,张慧宇1,马存根1,2,殷金珠3,宋丽娟2,3,尉杰忠1,2,4
- 1山西大同大学脑科学研究所/分子细胞免疫学大同市重点实验室/附属第一医院神经科,山西省大同市 037009;2山西中医药大学国家中医药管理局多发性硬化益气活血重点研究室/神经生物学研究中心,山西省晋中市 030619;3国药同煤总医院神经外科/山西省卫健委神经疾病防治研究重点实验室,山西省大同市 037003;4山西大同市第五人民医院,山西省大同市 037009
Astragaloside inhibits astrocyte activation and inflammatory response induced by inflammation
Yu Jingwen1, Guo Minfang1, Zhang Bingxin1, Mu Bingtao1, Meng Tao1, Zhang Huiyu1, Ma Cungen1, 2, Yin Jinzhu3, Song Lijuan2, 3, Yu Jiezhong1, 2, 4
- 1Institute of Brain Science/Key Laboratory of Molecular Cellular Immunology in Datong City/Department of Neurology of First Affiliated Hospital, Shanxi Datong University, Datong 037009, Shanxi Province, China; 2Key Research Laboratory of Benefiting Qi for Acting Blood Circulation Method to Treat Multiple Sclerosis of State Administration of Traditional Chinese Medicine/Research Center of Neurobiology, Shanxi University of Chinese Medicine, Jinzhong 030619, Shanxi Province, China;
摘要:
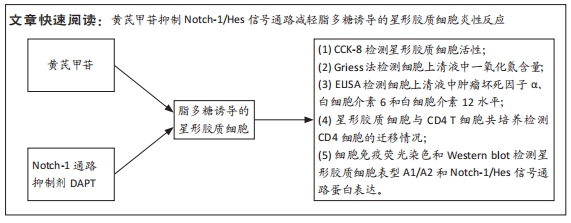
文题释义:
黄芪甲苷:是从传统益气中药黄芪中提取出来的具有生物活性成分的单体,是评价黄芪药材质量优劣的标准。课题组前期研究发现黄芪甲苷对中枢神经系统具有免疫调节、抗炎、抗氧化、抗凋亡以及保护线粒体等功能,可以促进神经的修复和再生。星形胶质细胞:是中枢神经系统中最丰富的一类胶质细胞,生理状态下具有营养、支持和代谢功能。近年研究发现,星形胶质细胞也参与免疫调节。在炎性环境下,星形胶质细胞被激活,会分化为A1和A2两个亚型,A1型会释放炎性因子,导致神经元退行性病变,A2型可以释放神经营养因子,对神经元起保护作用,但炎症激活下A1型要多于A2型,两者失衡会导致许多疾病:如缺血性脑卒中、帕金森症、阿尔茨海默症等。研究炎性环境下星形胶质细胞的表型改变对于了解这些疾病具有重要的意义。
背景:星形胶质细胞在中枢神经系统疾病的病理中起着重要作用。星形胶质细胞的表型变化及功能改变,提示其可能是中枢神经系统疾病的有效治疗靶点。前期研究证实,黄芪甲苷可以抑制脂多糖(lipopolysaccharide,LPS)诱导的星形胶质细胞炎性反应,其是否可以通过Notch-1及其下游信号通路调控星形胶质细胞表型和功能,尚不清楚。
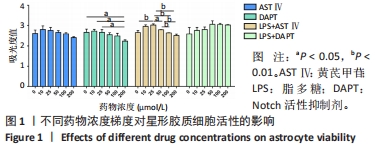
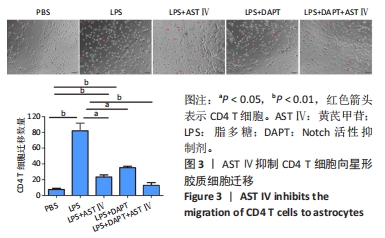
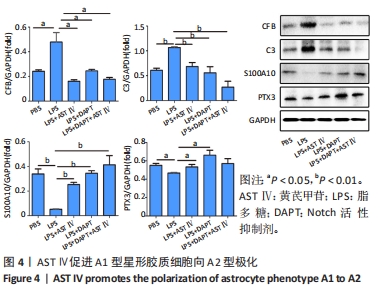
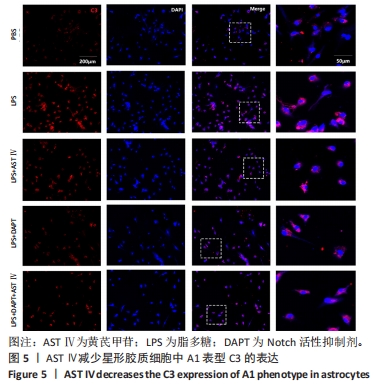
目的:探讨黄芪甲苷对炎性诱导的星形胶质细胞激活及炎症反应的影响及可能机制。方法:体外培养新生C57BL/6小鼠大脑皮质星形胶质细胞,CCK-8法检测星形胶质细胞活力确定黄芪甲苷及Notch活性抑制剂DAPT的最适浓度;然后将星形胶质细胞分为5组:PBS组、LPS组、LPS+黄芪甲苷组、LPS+DAPT组和LPS+DAPT+黄芪甲苷组,ELISA法检测炎性因子分泌水平,Griess法检测一氧化氮水平,用Transwell小室将星形胶质细胞与脾脏单个核细胞共培养,观察CD4 T细胞的迁移情况,免疫荧光染色检测星形胶质细胞活化标志物GFAP、星形胶质细胞的A1标记物C3、A2标记物S100A10以及信号通路相关Notch-1、Jag-1的表达,Western blot法检测CFB、C3、S100A10、PTX3、Notch-1、Jag-1和Hes的表达。
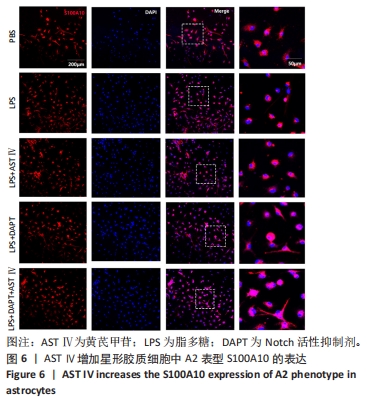
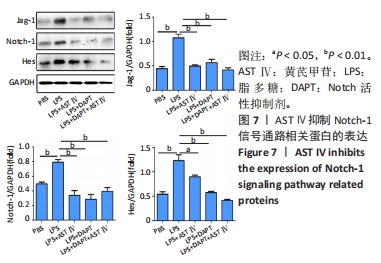
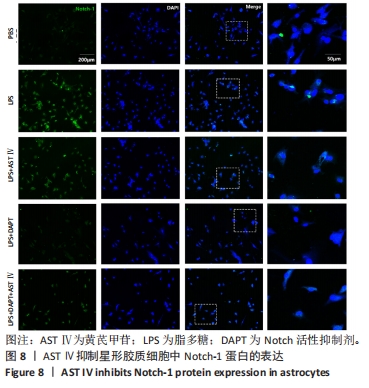
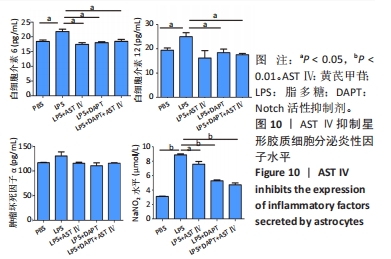
结果与结论:①根据CCK-8结果,筛选黄芪甲苷终浓度为25 μmol/L,DAPT终浓度为50 μmol/L进行后续实验;②与PBS组相比,LPS组白细胞介素6、白细胞介素12和一氧化氮分泌水平显著升高(P < 0.05,P < 0.05,P < 0.01);与LPS组相比,LPS+黄芪甲苷组、LPS+DAPT组、LPS+DAPT+黄芪甲苷组白细胞介素6(均为P < 0.05)、白细胞介素12(P > 0.05,P < 0.05,P < 0.05)和一氧化氮(P < 0.05,P < 0.01,P < 0.01)分泌显著减少;③与PBS组相比,LPS组星形胶质细胞激活明显,GFAP表达明显增多,CD4 T细胞的迁移数量明显增多(P < 0.01);与LPS组相比,LPS+黄芪甲苷组、LPS+DAPT组、LPS+DAPT+黄芪甲苷组星形胶质细胞激活受到显著抑制,CD4 T细胞迁移减少(P < 0.05,P < 0.05,P < 0.01);④与PBS组相比,LPS组A1型标记物C3和CFB的表达增加(P < 0.01,P < 0.05),而A2型标记物S100A10和PTX3的表达减少(P < 0.01,P < 0.05);与LPS组相比,LPS+黄芪甲苷组、LPS+DAPT组、LPS+DAPT+黄芪甲苷组C3(均为P < 0.01)和CFB(均为P < 0.05)的表达显著减少,S100A10(均为P < 0.01)和PTX3(P < 0.05,P < 0.05和P > 0.05)的表达增加;⑤与PBS组相比,LPS组Jag-1、Notch-1和Hes表达明显增加(均为P < 0.01);与LPS组相比,LPS+黄芪甲苷组、LPS+DAPT组、LPS+DAPT+黄芪甲苷组Jag-1(均为P < 0.01)、Notch-1(均为P < 0.01)和Hes(P < 0.05,P < 0.01,P < 0.01)表达显著减少;⑥结果表明:黄芪甲苷可通过调控Notch-1信号通路促进星形胶质细胞由A1型向A2型转化,减少炎性因子的分泌和CD4 T细胞的迁移,从而抑制星形胶质细胞的激活和炎性反应。
https://orcid.org/0000-0002-3983-7954 (于婧文)
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
中图分类号: