中国组织工程研究 ›› 2024, Vol. 28 ›› Issue (31): 5010-5016.doi: 10.12307/2024.713
• 干细胞基础实验 basic experiments of stem cells • 上一篇 下一篇
吲哚丙酸抑制小胶质细胞M1极化治疗脊髓损伤
滕益霖1,席德双1,冯彦斌1,梁 宇1,2,邓 豪3,曾高峰4,宗少晖1
- 1广西医科大学第一附属医院脊柱骨病外科,广西壮族自治区南宁市 530000;2广西医科大学第三附属医院脊柱骨病外科,广西壮族自治区南宁市 530000;广西医科大学,3再生医学与医用生物资源开发应用省部共建协同创新中心,4公共卫生学院,广西壮族自治区南宁市 530000
Indolepropionic acid inhibition of microglial cell M1 polarization for treatment of spinal cord injury
Teng Yilin1, Xi Deshuang1, Feng Yanbin1, Liang Yu1, 2, Deng Hao3, Zeng Gaofeng4, Zong Shaohui1
- 1Department of Spine Surgery, First Affiliated Hospital of Guangxi Medical University, Nanning 530000, Guangxi Zhuang Autonomous Region, China; 2Department of Spine Surgery, Third Affiliated Hospital of Guangxi Medical University, Nanning 530000, Guangxi Zhuang Autonomous Region, China; 3Collaborative Innovation Center of Regenerative Medicine and Medical Bioresource Development and Application Co-constructed by the Province and Ministry, Guangxi Medical University, Nanning 530000, Guangxi Zhuang Autonomous Region, China; 4School of Public Health, Guangxi Medical University, Nanning 530000, Guangxi Zhuang Autonomous Region, China
摘要:
文题释义:
M1表型小胶质细胞:可释放大量的肿瘤坏死因子α、白细胞介素1β、诱导型一氧化氮合酶、一氧化氮等神经毒性物质,导致脊髓损伤后炎症反应的持续发展,并进一步损害脊髓组织。吲哚丙酸:一种肠道菌群色氨酸代谢产物,被证实可减轻中枢神经系统炎症、肠道炎症环境及促进周围神经损伤后轴突再生。
背景:吲哚丙酸被证明可减轻糖尿病所致的中枢神经系统炎症,但其能否抑制小胶质细胞M1极化治疗脊髓损伤,目前仍缺乏相关研究。
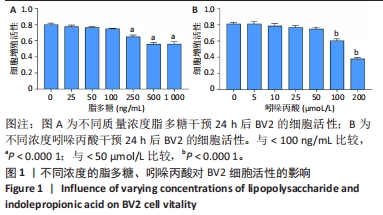
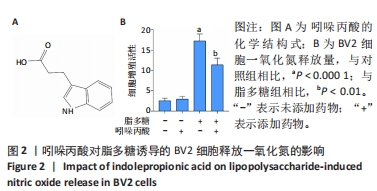
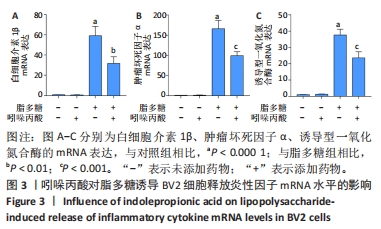
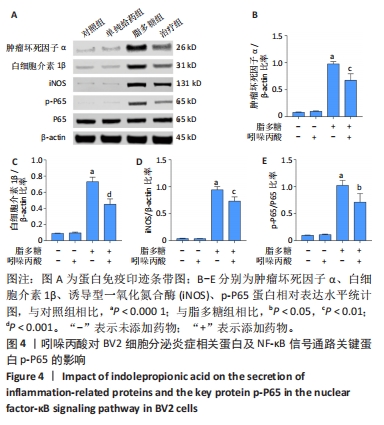
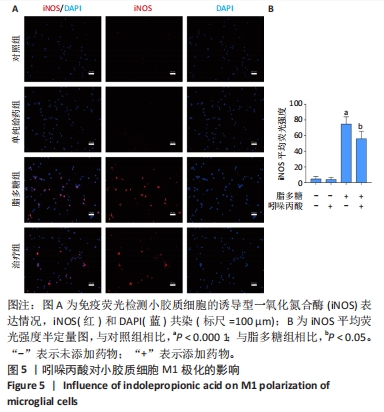
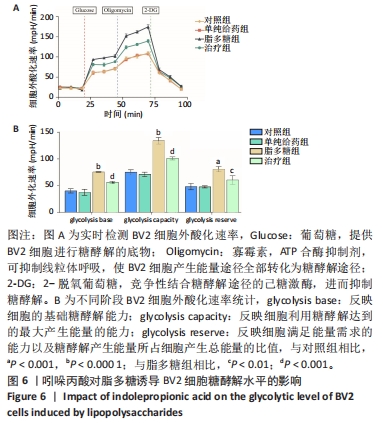
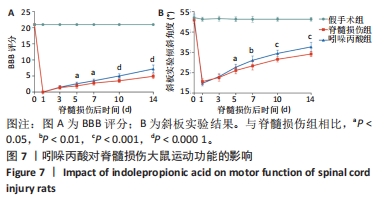
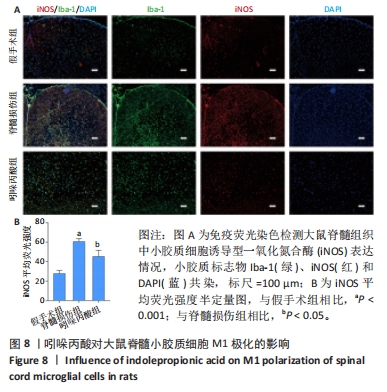
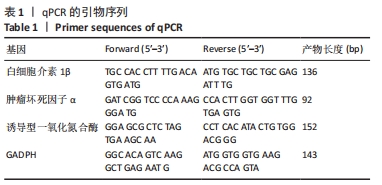
目的:通过细胞实验及动物实验探究吲哚丙酸抑制小胶质细胞M1极化治疗脊髓损伤的机制。方法:①体外实验:CCK8法检测BV2细胞活性并筛选最佳的吲哚丙酸使用浓度;然后将BV2细胞分为对照组、单纯给药组(50 μmol/L吲哚丙酸)、脂多糖组(100 ng/mL脂多糖)、治疗组(100 ng/mL脂多糖+50 μmol/L吲哚丙酸),采用 Griess 法检测一氧化氮含量;实时荧光定量PCR、Western Blot检测促炎相关因子的mRNA和蛋白的表达;细胞免疫荧光染色检测诱导型一氧化氮合酶的表达;Seahorse实验检测BV2细胞糖酵解压力水平。②体内实验:将30只SD大鼠随机分成3组:假手术组、脊髓损伤组、吲哚丙酸组。采用BBB评分与斜板实验评估大鼠脊髓损伤后功能恢复情况;免疫荧光染色检测脊髓组织中小胶质细胞诱导型一氧化氮合酶的表达情况;ELISA检测脊髓组织中促炎因子白细胞介素1β及肿瘤坏死因子α蛋白表达水平。
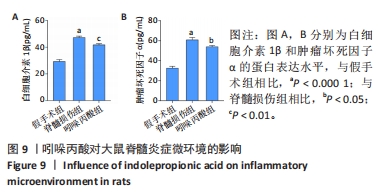
结果与结论:①体外实验:当吲哚丙酸浓度> 50 μmol/L时明显抑制BV2细胞活性;吲哚丙酸可通过抑制核因子κB信号通路的激活,进而抑制促炎因子(白细胞介素1β、肿瘤坏死因子α)及BV2细胞M1极化标志物诱导型一氧化氮合酶的mRNA及蛋白表达水平;吲哚丙酸能明显降低脂多糖诱导BV2细胞的糖酵解水平。②体内实验:脊髓损伤大鼠给予吲哚丙酸干预后,BBB评分与斜板实验角度明显增加;脊髓组织中M1极化小胶质细胞的数量显著下降及促炎因子(白细胞介素1β、肿瘤坏死因子α)蛋白表达水平明显降低。③结果说明,吲哚丙酸可通过抑制小胶质细胞M1极化改善炎症微环境促进脊髓损伤后功能恢复。
https://orcid.org/0009-0005-2265-1159 (滕益霖)
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
中图分类号: