中国组织工程研究 ›› 2024, Vol. 28 ›› Issue (25): 4034-4040.doi: 10.12307/2024.180
• 干细胞基础实验 basic experiments of stem cells • 上一篇 下一篇
骨形态发生蛋白4诱导下骨骼肌内异位骨化的细胞来源
余洋溢,廉 强,吴建群,张 轩,任晋可,李广恒
- 深圳市肌肉骨骼组织重建与功能恢复重点实验室,关节骨科,深圳市人民医院(暨南大学第二临床医学院,南方科技大学第一附属医院) ,广东省深圳市 510515
-
收稿日期:2023-04-27接受日期:2023-04-28出版日期:2024-09-08发布日期:2023-11-24 -
通讯作者:李广恒,博士,主任医师,深圳市肌肉骨骼组织重建与功能恢复重点实验室,关节骨科,深圳市人民医院(暨南大学第二临床医学院,南方科技大学第一附属医院) ,广东省深圳市 510515 -
作者简介:余洋溢,男,1987年生,河南省人,汉族,2019年郑州大学毕业,博士,主治医师,主要从事骨关节退行性疾病、运动损伤的科研与临床工作。 廉强,深圳市肌肉骨骼组织重建与功能恢复重点实验室,关节骨科,深圳市人民医院(暨南大学第二临床医学院,南方科技大学第一附属医院) ,广东省深圳市 510515 -
基金资助:国家自然科学基金面上项目(81472136,82172463),项目负责人:李广恒
Cell-of-origin for heterotopic ossification induced by bone morphogenetic protein 4 in skeletal muscle
Yu Yangyi, Lian Qiang, Wu Jianqun, Zhang Xuan, Ren Jinke, Li Guangheng
- Shenzhen Key Laboratory of Musculoskeletal Tissue Reconstruction and Function Restoration, Department of Adult Joint Reconstruction and Orthopedic Surgery, Shenzhen People’s Hospital (Second Clinical Medical College of Jinan University, First Affiliated Hospital, Southern University of Science and Technology), Shenzhen 510515, Guangdong Province, China
-
Received:2023-04-27Accepted:2023-04-28Online:2024-09-08Published:2023-11-24 -
Contact:Li Guangheng, PhD, Chief physician, Shenzhen Key Laboratory of Musculoskeletal Tissue Reconstruction and Function Restoration, Department of Adult Joint Reconstruction and Orthopedic Surgery, Shenzhen People’s Hospital (Second Clinical Medical College of Jinan University, First Affiliated Hospital, Southern University of Science and Technology), Shenzhen 510515, Guangdong Province, China -
About author:Yu Yangyi, PhD, Attending physician, Shenzhen Key Laboratory of Musculoskeletal Tissue Reconstruction and Function Restoration, Department of Adult Joint Reconstruction and Orthopedic Surgery, Shenzhen People’s Hospital (Second Clinical Medical College of Jinan University, First Affiliated Hospital, Southern University of Science and Technology), Shenzhen 510515, Guangdong Province, China Lian Qiang, Shenzhen Key Laboratory of Musculoskeletal Tissue Reconstruction and Function Restoration, Department of Adult Joint Reconstruction and Orthopedic Surgery, Shenzhen People’s Hospital (Second Clinical Medical College of Jinan University, First Affiliated Hospital, Southern University of Science and Technology), Shenzhen 510515, Guangdong Province, China -
Supported by:General Project of National Natural Science Foundation of China, Nos. 81472136, 82172463 (to LGH)
摘要:
文题释义:
异位骨化:指在正常情况下不具有骨化性质的组织中的骨形成,包括继发于肌肉、骨骼损伤后的异位骨化、神经源性异位骨化和进行性纤维发育不良性骨化等。其主要特点是在软组织中钙化骨迅速形成,于伤后3-12周形成异位骨组织,引起关节周围肿胀、疼痛及关节活动障碍等症状,甚至出现周围神经嵌压和压迫性溃疡。人骨形态发生蛋白4:作为骨形态发生蛋白家族成员之一,此蛋白在空间结构上与骨形态发生蛋白大体结构有一致性,只有在成熟区序列方面有其特异性。骨形态发生蛋白4在促进骨组织再生修复方面发挥着重要作用。此外,骨形态发生蛋白4也与诱导胚胎分化、指导神经干细胞分化、调节肿瘤生长侵袭以及一些心脑血管疾病密切相关。
背景:骨骼肌异位骨化是临床上严重的并发症。对于骨骼肌异位骨化而言,其参与成骨过程中的细胞仍不明确。
目的:观察肌细胞及筋膜细胞以及内皮细胞在骨肌中异位骨化过程中的参与情况,观察骨形态发生蛋白4诱导下骨骼肌内异位骨化的细胞来源。方法:培养C2C12细胞和诱导培养基数天下C2C12细胞形成的肌管,将质量浓度500 ng/mL 骨形态发生蛋白4分别加入培养基后,显微镜下观察处理10 d内 C2C12细胞和肌管是否继续增殖;按不同比例共培养大鼠肌细胞(L6)和人成纤维源性细胞(fibroblast-derived cells,FDC),通过番红O染色和阿尔新蓝染色研究上述细胞在质量浓度500 ng/mL的骨形态发生蛋白4和质量浓度10 ng/mL的转化生长因子β3处理下21 d内成骨和成软骨分化潜力。使用转基因动物FVB/N-TgN(TIE2-LacZ)182Sato小鼠,通过在基因鼠大腿肌间隙植入含有15 μL的腺相关病毒-骨形态发生蛋白4 (5×1010 PFU/mL)10 d及14 d,再通过X-gal 染色来观察异化骨中有无新血管内皮生成。
结果与结论:①骨形态发生蛋白4导致肌束退化并增加C2C12细胞增殖。与其他组相比,FDC组具有较高的阿尔新蓝和番红O染色面积(P < 0.05)和较低碱性磷酸酶染色面积(P < 0.05);而L6组和其他组相比具有更大的碱性磷酸酶染色面积(P < 0.05),但阿尔新蓝和番红O染色面积较小(P < 0.05)。②将腺相关病毒-骨形态发生蛋白4吸附的明胶海绵移植到FVB/N-TgN (TIE2-LacZ) 182Sato小鼠中会导致异位骨化。③X-gal染色结果显示,在软骨细胞及异化骨中无明显染色,提示Tie2+内皮细胞不参与异位骨化的形成。④结果证实,在腺相关病毒-骨形态发生蛋白4诱导的骨骼肌异位软骨化过程中,成纤维细胞是软骨细胞的主要细胞来源,而肌源性细胞是成骨细胞的主要来源。Tie2+内皮细胞可能不是软骨和骨的细胞来源。
https://orcid.org/0000-0003-0292-0954 (余洋溢)
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
中图分类号:
引用本文
余洋溢, 廉 强, 吴建群, 张 轩, 任晋可, 李广恒. 骨形态发生蛋白4诱导下骨骼肌内异位骨化的细胞来源[J]. 中国组织工程研究, 2024, 28(25): 4034-4040.
Yu Yangyi, Lian Qiang, Wu Jianqun, Zhang Xuan, Ren Jinke, Li Guangheng. Cell-of-origin for heterotopic ossification induced by bone morphogenetic protein 4 in skeletal muscle[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(25): 4034-4040.
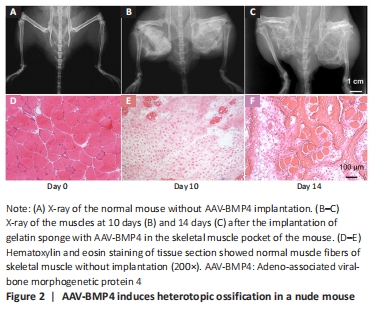
The gelatin sponge adsorbed with the 15 μL AAV-BMP4 virus solution was embedded in the skeletal muscle pocket of the nude mouse bilaterally. In 2 weeks, the heterotopic ossification was established locally (Figure 2A–C). The process of heterotopic ossification was observed through endochondral bone formation (Figure 2D–F).
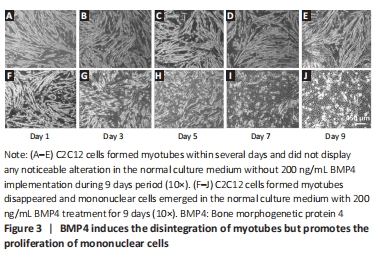
BMP4 induces the disintegration of multinuclear myotubes and promotes the proliferation of mononuclear myogenic cells
After C2C12 formed myotubes on Day 9 (Figure 3A–E), BMP4 treatment led to the disintegration of multinuclear myotubes, but promoted the proliferation of mononuclear cells (Figure 3F–J, Figure 4). In addition, according to our previous studies, BMP4 also promotes the osteogenesis of myogenic cells[23].
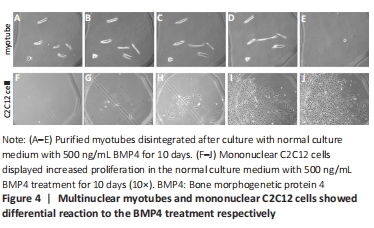
Firborgenic cells displayed more prone to chondrogenic differentiation, while myognic cells showed more readily to osteogenic differentiation
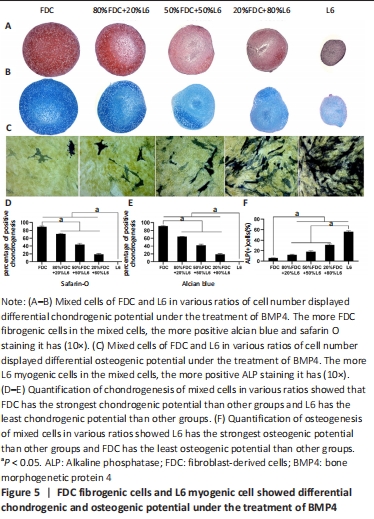
As shown in Figure 5A–B, mixed cells of FDC and L6 in various ratios of cell number displayed differential chondrogenic potential under the treatment of BMP4. The more FDC fibrogenic cells in the mixed cells, the more positive alcian blue and safarin O staining it had (Figure 5D–E). Mixed cells of FDC and L6 in various ratios of cell number displayed differential osteogenic potential under the treatment of BMP4. As shown in Figure 5C–F, the more L6 myogenic cells in the mixed cells, the more positive ALP staining it had.
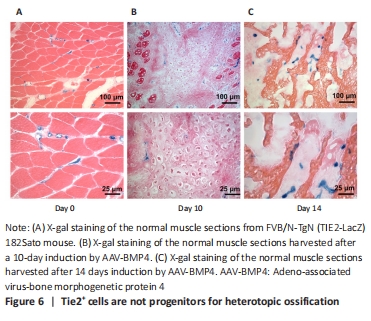
Tie2+ cells are not progenitors for heterotopic ossification in our study
Next, we investigated the fate of endothelial cells during heterotopic ossification. Tie2+ is predominantly expressed by vascular endothelial cells, and is widely used as a marker for endothelial cells[23]. Tie2-Cre transgenic mice have been used by several scientists for lineage tracing to understand the progression of heterotopic ossification[24]; they reported that Tie2+ cells could differentiate into chondrocytes or osteocytes during heterotopic ossification induced by BMP4. However, different conclusions were reached in our study. In our experiment, FVB/N-TgN (TIE2-LacZ) 182Sato mice was purchased from The Jackson Laboratory (Bar Harbor, ME) and used express β-galactosidase driven by the endothelial-specific TIE2 promoter. As Tie2-Cre transgenic mice were reported to have nonspecific recombination, the FVB/N-TgN (TIE2-LacZ) 182Sato mouse was used to study the role of Tie2+ cells in the heterotopic ossification process. We embedded the AAV-BMP4 sponge into the muscle of the FVB/N-TgN (TIE2-LacZ) 182Sato transgenic mice. On days 10 and 14, the ectopic bone formed in the muscle was harvested for X-gal staining (Figure 6). Without AAV-BMP4 injection, endothelial cells were distributed between the myofibers (Figure 6A). Round staining indicated that these Tie2+ cells were distributed in the blood vessel. After 10 days of induction, chondrocytes were observed in the muscles (Figure 6B). However, these chondrocytes were not stained with X-gal, indicating that Tie2+ cells were not the original cells for chondrocytes in heterotopic ossification. However, we observed X-gal-stained cells along the chondrocytes and between the myofibers. After 14 days of induction, bone trabeculae were observed (Figure 6C); however, there were no Tie2+ cells present, although stained cells were observed in the bone marrow-like region. Overall, using the transgenic mouse, we found that endothelial cells, which were Tie2 positive, were not progenitors for heterotopic ossification, but were involved in the process.
| [1] MEYERS C, LISIECKI J, MILLER S, et al. Heterotopic ossification: a comprehensive review. JBMR Plus. 2019;3(4):e10172. [2] YANG YS, KIM JM, XIE J, et al. Suppression of heterotopic ossification in fibrodysplasia ossificans progressiva using AAV gene delivery. Nat Commun. 2022; 13(1):6175. [3] KAN C, DING N, YANG J, et al. BMP-dependent, injury-induced stem cell niche as a mechanism of heterotopic ossification. Stem Cell Res Ther. 2019;10(1):14. [4] LEES-SHEPARD JB, STOESSEL SJ, CHANDLER JT, et al. An anti-ACVR1 antibody exacerbates heterotopic ossification by fibro-adipogenic progenitors in fibrodysplasia ossificans progressiva mice. J Clin Invest. 2022;132(12):e153795. [5] TSANG KY, CHEAH KS. The extended chondrocyte lineage: implications for skeletal homeostasis and disorders. Curr Opin Cell Biol. 2019;61:132-140. [6] HUANG Y, WANG X, ZHOU D, et al. Macrophages in heterotopic ossification: from mechanisms to therapy. NPJ Regen Med. 2021;6(1):70. [7] SHAHRIYARI M, ISLAM MR, SAKIB SM, et al. Engineered skeletal muscle recapitulates human muscle development, regeneration and dystrophy. J Cachexia Sarcopenia Muscle. 2022;13(6):3106-3121. [8] FENG H, XING W, HAN Y, et al. Tendon-derived cathepsin K-expressing progenitor cells activate Hedgehog signaling to drive heterotopic ossification. J Clin Invest. 2020;130(12):6354-6365. [9] ZHANG Q, ZHOU D, WANG H, et al. Heterotopic ossification of tendon and ligament. J Cell Mol Med. 2020;24(10):5428-5437. [10] EGHBALI-FATOURECHI GZ, LAMSAM J, FRASER D, et al. Circulating osteoblast-lineage cells in humans. N Engl J Med. 2005;352(19):1959-1966. [11] NESTI LJ, JACKSON WM, SHANTI RM, et al. Differentiation potential of multipotent progenitor cells derived from war-traumatized muscle tissue. J Bone Joint Surg Am. 2008;90(11):2390-2398. [12] UEZUMI A, FUKADA S, YAMAMOTO N, et al. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol. 2010;12(2):143-152. [13] DROUIN G, COUTURE V, LAUZON MA, et al. Muscle injury-induced hypoxia alters the proliferation and differentiation potentials of muscle resident stromal cells. Skelet Muscle. 2019;9(1):18. [14] LAVASANI M, LU A, THOMPSON SD, et al. Isolation of muscle-derived stem/progenitor cells based on adhesion characteristics to collagen-coated surfaces. Methods Mol Biol. 2013;976:53-65. [15] TU B, YU B, WANG W, et al. Inhibition of IL-17 prevents the progression of traumatic heterotopic ossification. J Cell Mol Med. 2021;25(16):7709-7719. [16] FELIX-ILEMHENBHIO F, PICKERING GAE, KISS-TOTH E, et al. Pathophysiology and emerging molecular therapeutic targets in heterotopic ossification. Int J Mol Sci. 2022;23(13):6983. [17] WOSCZYNA MN, BISWAS AA, COGSWELL CA, et al. Multipotent progenitors resident in the skeletal muscle interstitium exhibit robust BMP-dependent osteogenic activity and mediate heterotopic ossification. J Bone Miner Res. 2012; 27(5):1004-1017. [18] LEES-SHEPARD JB, YAMAMOTO M, BISWAS AA, et al. Activin-dependent signaling in fibro/adipogenic progenitors causes fibrodysplasia ossificans progressiva. Nat Commun. 2018;9(1):471. [19] SHEN F, SHI Y. Recent advances in single-cell view of mesenchymal stem cell in osteogenesis. Front Cell Dev Biol. 2021;9:809918. [20] CAPLAN AI. Mesenchymal stem cells. J Orthop Res. 1991;9(5):641-650. [21] LI G, PENG H, CORSI K, et al. Differential effect of BMP4 on NIH/3T3 and C2C12 cells: implications for endochondral bone formation. J Bone Miner Res. 2005; 20(9):1611-1623. [22] LI G, ZHENG B, MESZAROS LB, et al. Identification and characterization of chondrogenic progenitor cells in the fascia of postnatal skeletal muscle. J Mol Cell Biol. 2011;3(6):369-377. [23] WERLEIN C, ACKERMANN M, STARK H, et al. Inflammation and vascular remodeling in COVID-19 hearts. Angiogenesis. 2023;26(2):233-248. [24] PRADOS B, DEL TORO R, MACGROGAN D, et al. Heterotopic ossification in mice overexpressing Bmp2 in Tie2+ lineages. Cell Death Dis. 2021;12(8):729. [25] CHAN NT, LEE MS, WANG Y, et al. CTR9 drives osteochondral lineage differentiation of human mesenchymal stem cells via epigenetic regulation of BMP-2 signaling. Sci Adv. 2022;8(46):eadc9222. [26] ALFAQIH MS, TARAWAN VM, SYLVIANA N, et al. Effects of vitaminutes d on satellite cells: a systematic review of in vivo studies. Nutrients. 2022;14(21):4558. [27] ROSINA M, LANGONE F, GIULIANI G, et al. Osteogenic differentiation of skeletal muscle progenitor cells is activated by the DNA damage response. Sci Rep. 2019; 9(1):5447. [28] RAUCH A, MANDRUP S. Transcriptional networks controlling stromal cell differentiation. Nat Rev Mol Cell Biol. 2021;22(7):465-482. [29] CONTRERAS O, ROSSI FMV, THERET M. Origins, potency, and heterogeneity of skeletal muscle fibro-adipogenic progenitors-time for new definitions. Skelet Muscle. 2021;11(1):16. [30] ALESSI WOLKEN DM, IDONE V, HATSELL SJ, et al. The obligatory role of activin a in the formation of heterotopic bone in fibrodysplasia ossificans progressiva. Bone. 2018;109:210-217. |
| [1] | 杨玉芳, 杨芷姗, 段棉棉, 刘毅恒, 唐正龙, 王 宇. 促红细胞生成素在骨组织工程中的应用及前景[J]. 中国组织工程研究, 2024, 28(9): 1443-1449. |
| [2] | 魏 娟, 李 婷, 郇梦婷, 谢 颖, 谢舟煜, 韦庆波, 吴云川. 静力性训练改善2型糖尿病骨骼肌胰岛素抵抗的机制[J]. 中国组织工程研究, 2024, 28(8): 1271-1276. |
| [3] | 王 继, 张 敏, 李文博, 杨中亚, 张 龙. 有氧运动对2型糖尿病大鼠糖脂代谢、骨骼肌炎症和自噬的影响[J]. 中国组织工程研究, 2024, 28(8): 1200-1205. |
| [4] | 孔健达, 穆玉晶, 朱 磊, 李志林, 陈世娟. 骨骼肌再生过程中卫星细胞调控机制及其生态位信号的作用[J]. 中国组织工程研究, 2024, 28(7): 1105-1111. |
| [5] | 王 雯, 郑芃芃, 孟浩浩, 刘 浩, 袁长永. 过表达Sema3A促进牙髓干细胞和MC3T3-E1的成骨分化[J]. 中国组织工程研究, 2024, 28(7): 993-999. |
| [6] | 杨毅峰, 黄 健, 叶 楠, 王 琳. 全膝关节置换中的缺血再灌注损伤[J]. 中国组织工程研究, 2024, 28(6): 955-960. |
| [7] | 申飞燕, 姚吉祥, 苏珊珊, 赵忠民, 唐巍东. 敲低环状RNA WD重复含蛋白1抑制膝骨关节炎软骨细胞增殖并诱导凋亡[J]. 中国组织工程研究, 2024, 28(4): 499-504. |
| [8] | 刘志杨, 傅泽铤, 夏 雨, 丁海丽. 运动性骨骼肌损伤中时钟基因BMAL1与MyoD的作用[J]. 中国组织工程研究, 2024, 28(4): 510-515. |
| [9] | 乔虎军, 王国祥. 白细胞介素1β诱导大鼠骨性关节炎软骨细胞模型的效果评价[J]. 中国组织工程研究, 2024, 28(4): 516-521. |
| [10] | 韦沅汛, 陈 锋, 林宗汉, 张 驰, 潘成镇, 韦宗波. Notch信号通路与骨质疏松症及中医药防治[J]. 中国组织工程研究, 2024, 28(4): 587-593. |
| [11] | 朱志祺, 苑思杰, 张梓琳, 纪仕杰, 蒙明松, 颜安明, 韩 婧. 六味地黄丸对钛颗粒诱导骨溶解成骨影响的机制[J]. 中国组织工程研究, 2024, 28(3): 392-397. |
| [12] | 张树东, 黄一琳, 姚 琦. 安石榴苷促进成骨治疗绝经后骨质疏松[J]. 中国组织工程研究, 2024, 28(26): 4101-4105. |
| [13] | 杨宗睿, 葛海雅, 石金玉, 汪正明, 王媛媛, 李正言, 杜国庆, 詹红生. “筋出槽”大鼠模型骨骼肌形态学和功能的特征性变化[J]. 中国组织工程研究, 2024, 28(26): 4170-4177. |
| [14] | 罗善超, 唐继仁. 橙皮素抑制氧化应激影响软骨细胞的炎性退变[J]. 中国组织工程研究, 2024, 28(26): 4184-4188. |
| [15] | 李家乐, 罗达胜, 郑刘杰, 刘 伟, 姚运峰. 人骨关节炎软骨细胞上调成骨细胞中骨保护素的作用途径[J]. 中国组织工程研究, 2024, 28(26): 4194-4201. |
Skeletal muscles are mainly made up of myofibers, satellite cells (myogenic cells), perimysium and endomysium cells (fibrogenic cells), endothelial cells, and nerve cells[7]. To date, various types of cells have been reported as the original cells for ectopic bone formation[8-9]. There is still no conclusion about the function of different types of cells involved in heterotopic ossification. Over the past few decades, animal studies have suggested that various cell types, including muscle-derived stem cells, circulating osteogenic precursor cells, and fibrous/adipogenic progenitor cells, are potential sources of HO progenitors[10-12]. Several studies have reported the presence of multiple stem cell populations, specifically muscle-derived stem cells, within skeletal muscle, which significantly contribute to the initiation and progression of traumatic HO. Additionally, muscle-derived stem cells isolated from injured muscle have shown greater sensitivity to osteogenic signals compared to those isolated from healthy muscle, indicating that post-injury hypoxia may play a crucial role in triggering HO in the microenvironment of muscle injuries[9-13].
It has been discovered that fibrous/adipogenic progenitor cells and multiple stem cells within skeletal muscle possess similar characteristics and share functional similarities. In a study conducted by LAVASANI et al.[14], muscle-derived stem cells and fibrous/adipogenic progenitor cells were separated based on their adherence to type I collagen-coated surfaces. The study observed that muscle-derived stem cells exhibited slower adhesion to type I collagen-coated surfaces compared to fibrous/adipogenic progenitor cells. However, the precise differentiation and identification between muscle-derived stem cells and fibrous/adipogenic progenitor cells continue to be a subject of controversy. The mesenchymal progenitor cell population plays a significant role in the development of traumatic HO, and further investigation is required to understand the specific contributions of these two progenitor cell types in the pathogenesis of traumatic HO[15-16].
Furthermore, in addition to their fibrotic and adipogenic potential, fibrous/adipogenic progenitor cells have also been reported to possess osteogenic potential[12-17]. WOSCZYNA et al.[17] found that FAP was the major contributor to BMP-induced HO, and only the PDGFRα+Sca-1+fraction of FAP exhibited osteogenic properties fibrous/adipogenic progenitor cells represents the primary cellular origin of hereditary HO, specifically fibrous dysplasia ossificans progressive[18-19].
Despite years of extensive research, the exact cellular origin of HO has not been fully elucidated. Although the contribution of mesenchymal cells to HO lesions has been demonstrated, there is still controversy surrounding whether these osteoblasts originate from tissue-resident cells or circulating mesenchymal cells. Scholars conducted a study that verified the mutation of the BMP4 gene rs17563C > T site through genetic sequencing, which led to significant overexpression of the BMP4 protein, promoting the occurrence of ossification of the posterior longitudinal ligament in the cervical spine, which explored the possible mechanisms of ectopic ossification of the posterior longitudinal ligament (collagen fibers)[20]. This article also involves the role of fascia (collagen fibers) in BMP4-induced osteogenesis. Yet, this paper primarily focuses on discussing the main cellular sources responsible for the production of ectopic ossification under BMP4 induction.
We hypothesized that fibrogenic cells in skeletal muscle are the primary source of progenitors that drive the heterotopic cartilage phase, while myogenic cells contribute mostly to the bone phase. Endothelial cells provide no contribution to both cartilage and bone phase during the heterotopic ossification. 中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
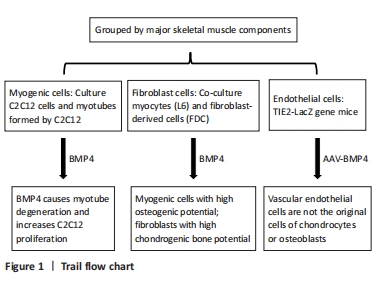
C2C12 formed myotubes and C2C12 myocytes were treated with BMP4; Analysis of osteogenic and chondrogenic differentiation potential of myocytes (L6) and fibroblast-derived cells (FDC) under BMP4 treatment; to study the effect of endothelial cell in HO induced by BMP4 in TIE2-LacZ gene mice (Figure 1).
The experiment was conducted in the Laboratory of Jinan University from January 2022 to January 2023.
Materials
Totally 6 female nude mice (4 weeks old, body weight 16–18 g) were purchased from the Vital River (Beijing, China) and allowed to grow for a further two weeks. Three female transgenic animal FVB/N-TgN (TIE2-LacZ) 182Sato mouse (4 weeks old, body weight 12–14 g) were donated by Professor Chao Liu (Southern University of Science and Technology, China). All animal research adhered to the institution’s or the National Research Council’s guidelines for the Care and Use of Laboratory Animals. This investigation was approved by the Institutional Animal Care and Use Committee of Shenzhen People’s Hospital at Jinan University in 2021 (approval No. LL-KY-2020023). C2C12 cells were purchased from the National Infrastructure of Cell Line Resource, a 6-mm × 6-mm piece of sterile gelatin sponge, and bovine serum albumin purchased from Sinopharm Chemical Reagent (Shenzhen, China).
Methods
Construction and production of adeno-associated virus (AAV)-BMP4
AAV-BMP4 was constructed, and after successful construction, it was implanted into the thigh muscle space of nude mice and transgenic mice. To assess the concentrations of the isolated virus particles, DNA dot-blot hybridization was used. Hybridizations were performed at 68 °C, according to the instructions of a non-radioactive DNA labeling and detection kit (Boehringer Mannheim), and at 75 °C or 80 °C.
The AAV-BMP4 virus packing cells were co-transfected with the traditional triple plasmids; the transfection details are as follows: Trypsinized 293 cells were grown into a 225-cm2 culture dish. The cell confluency of the monolayer culture ranged between 20% and 40%. To ensure equal cell dispersion across the dish, cell culture media (40 mL) were poured into the dish. When the cell confluence reached around 80%, cells were transfected. Half of the fresh cell culture media were replaced 1 hour before transfection, and 23 μg of the AAV plasmid of interest and two helper plasmids were added to 4 mL calcium chloride solution (300 mmol/L). The combined solutions were introduced to the dish containing the 293 cells in a consistent manner. The cell suspension was collected 3 days later and repeatedly frozen/thawed to obtain the viral vector.
The AAV virus particles were purified using a cesium chloride gradient and high-speed centrifugation. 11 mL of 40% sucrose solution containing 0.01% bovine serum albumin and 48 mL crude viral extract were put into a sterile ultrafiltration tube. For 16 hours, the mixture was centrifuged at 100,000 r/minutes at 4 °C. Cells were centrifuged repeatedly after the addition of cesium chloride until pure AAV virus particles were recovered.
Preparation of tissue samples
As described in previous studies[21-22], endothelial cells were activated by 1-week fluid shear stress stimulation. Therefore, in the present study, the rats were killed 1 week after operation. The gracilis muscle was isolated from each animal. According to the angiographic results, during the growth of collateral vessels, the gracilis muscle usually contained 2–4 collateral vessels. For immunofluorescence, all samples were embedded in OCT and stored at –80 °C refrigerator until use.
AAV-BMP4 administration in skeletal muscle of mice
After the animals were adequately anesthetized with a mixture of ketamine (90 mg/kg, intraperitoneally) and xylazine (10 mg/kg, intraperitoneally), a 5-mm sterile incision was made in hindlimbs. 10 pieces of Gelfoam (Pfizer, Kalamazoo, Michigan, 3 mm × 3 mm) soaked with 15 μL AAV-BMP4 (5 × 1010 PFU/mL) were implanted into the muscle pocket of the legs of nude mice. One interrupted 4–0 silk suture was used to close the incision. Animals were allowed ad libitum activity, food, and water after the injection. As described previously, the implanted mice were observed to observe by an X-ray mammography device (Kodak, Directview, DR3000) after surgery, with samples harvested 14 days after surgery[21]. The formation of heterotopic ossification in thigh skeletal muscle within 14 days was observed by X-ray, and the histological changes of heterotopic ossification were further observed by hematoxylin-eosin staining. The formation process of heterotopic ossification was qualitatively measured by selecting five different fields of view and measuring the proportion of chondrocytes and osteocytes to the total cells.
Cell culture
Cultivate and isolate cells for later use. C2C12 cells (purchased from the National Infrastructure of Cell Line Resource) were grown in T-75-cm2 flasks in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin and were incubated at 37 °C in humidified air mixed with 5% CO2. Cells were prevented from becoming 100% confluent and were trypsinized and passaged every 3–4 days.
Fresh skeletal muscle fascia tissue was carefully detached from the muscle using surgical scissors and then washed three times. The harvested fascia tissue was minced into pieces using scissors; the minced tissue was placed in a 15 mL centrifuge tube containing 3 mL of 0.2% collagenase for 1 hour and then incubated for 30 minutes with 0.1% trypsin at 37 °C. The digested tissue solution was filtered through a 100 mesh filter, and the filtrate was centrifuged at 900 r/minutes for 5 minutes. The supernatant was discarded, and the precipitated cells were suspended in DMEM with 10% fetal bovine serum, added to a 6-well plate, and cultured in a 37 °C cell incubator (Thermo Fisher, model: Steri-Cycle i160 CO2 incubator). Cell morphology was observed under a BX63F light microscope (Olympus Corporation, Japan).
Treatment of BMP4 on C2C12 cells and myotubes
The myotubes were cultured in a medium with and without BMP4, and the changes in myotubes were observed within 9 days. C2C12 cells were grown to 100% confluence. DMEM supplemented with 2% horse serum and 10 µm β-D-arabinofuranoside (ara-cytosine) was used to induce myotubes. After several days, 80% of the cells fused and differentiated into myotubes. C2C12 cells formed myotubes were co-cultured with and without BMP for 10 days, and the changes in myotubes were observed under a light microscope. Three fields of view were randomly selected to calculate the changes in the number of myotubes under the light microscope.
In addition, several myotube and several C2C12 cells were co-cultured with BMP4, and the number changes of myotube and cells were observed within 10 days. In addition, myotubes were detached using 0.05% trypsin after myotubes were obtained as above. The solution containing detached myotubes and C2C12 cells was gently passed through a 100-μm sieve. The myotubes on the sieve were transferred and cultured in a 0.75% gelatin-coated plate with DMEM supplemented with 0.5% fetal bovine serum. Purified myotubes were treated with 500 ng/mL BMP4 and C2C12 cells were used as controls under the same treatment. Images were acquired using BX63F light microscope (Olympus Corporation). Under the light microscope, we checked whether the C2C12 cells continued to proliferate in the culture dish and observe whether the myotube cells continued to proliferate. Three fields of view were randomly selected to calculate the changes in the shape of myotubes and the number of C2C12 cells under the light microscope.
Chondrogenic and osteogenesis assay for mixed pellets of fibroblast-derived cells (FDCs) and rat L6 myoblasts in vitro
Using a method described previously[22], FDCs and rat L6 myoblast cells were mixed and centrifuged to make pellets with different ratios (1:0, 4:1, 1:1, 1:4, 0:1; FDCs to L6 cells) with each pellet having the same total number of cells (2.5 × 105), and the resulting pellets were cultured for up to 28 days in chondrogenic medium (Cambrex) supplemented with 500 ng/mL BMP4 and 10 ng/mL transforming growth factor-β3 (R&D Systems, UK). The medium was changed every 2–3 days, and the pellets were harvested on day 21, embedded in paraffin, sectioned, and stained with Alcian blue/nuclear fast red (Sigma, USA). ALP staining was performed to reveal osteogenic differentiation of the mixed cells treated with BMP4[21]. Images were acquired using BX63F light microscope (Olympus Corporation). The proportion of corresponding positive cells was calculated by ImageJ software, and then the degree of osteogenic or chondrogenic differentiation of the two different cells was evaluated.
AAV-BMP4 administration in skeletal muscle of FVB/N-lacZ mice
Six mice were divided into a control group and an experimental group, wherein the control group was implanted with AAV without BMP4, and the experimental group contained AAV-BMP4. Six pieces of Gelfoam (Pfizer, Kalamazoo, Michigan, 3 × 3 mm2) soaked with 15 μL AAV-BMP4 (5 × 1010 PFU/mL) were implanted into the legs of nude mice. The implanted mice were observed to observe by an X-ray mammography device (Kodak, Directview, DR3000, USA) after surgery, and samples were harvested on days 10 and 14 after surgery.
X-gal staining
After raising the transgenic mice for 0, 10, and 14 days, the sections were stained respectively. Details are seen as follows: Fresh frozen tissue sections were fixed in cold 10% formalin (4 °C) for 10 minutes. Slides were washed with PBS three times, each time lasting 5 minutes, and then rinsed in distilled water. Slides were incubated in an X-gal working solution at 37 °C for 24 hours (using a humidified chamber to prevent slides from drying). Sections were rinsed in PBS for 10 minutes and then briefly rinsed with distilled water. Sections were counterstained with nuclear fast red for 3–5 minutes. Images were acquired using BX63F light microscope (Olympus Corporation, Japan). Chondrocytes and bone trabeculae were observed by whether staining with X-gal or not.
Main observation indicators
(1) Whether the myotube break down and C2C12 cells proliferate or not; (2) Compared with other groups, pure FDC group, and pure L6 group had more positive alcian blue and safarin O staining or more positive ALP staining under BMP4 treatment; (3) Investigation of whether X-gal staining is exit these chondrocytes or bone trabeculae in ectopic ossification under BMP4 induction in TIE2-LacZ transgenic mice.
Statistical analysis
All data were statistically processed using SPSS software (V 26.0, USA). The mean±SD was used to represent the data of each group, one-way variance for different time points within the group. The two sample t-test was utilized to compare the data between the two groups. P < 0.05 was considered as the difference to be significant. The statistical methods of the article had been reviewed by biostatistical experts from Jinan University.
C2C12 cell is a myogenic cell line which can fuse with each other and differentiate into multinuclear myotube by dropping culture serum in vitro. As the representative of myofibers in skeletal muscle, C2C12 cells formed myotube were tested under the BMP4 treatment in vitro. Previous in vitro and in vivo studies concluded that Bmp is a potent suppressor of skeletal differentiation in all muscle progenitors[25]. Consistant with the studies, our study demonstrated that C2C12 fused myotubes disintegrated and mononuclear cells emerged when treated by BMP4 protein for a certain of time when cultured in vitro. Further experiment was carried out to understand this observation, C2C12 fused myotubes were purified and cultured in vitro with comparison to single mononuclear C2C12 cells. Under the treatment of BMP4, purified myotubes degenerated and mononuclear C2C12 cells proliferated, which confirming the results of the previous literature that Bmp4 exposure increased the number of satellite cell[26]. As for the osteogenic effect of myogenic cells, some studies have doubted it[27]. However, in this paper, pure myogenic cell cultures showed osteogenic efficiency. Combined with previous studies, we give a positive answer to whether myogenic cells have osteogenic efficiency[28].
Fibrogenic cells isolated from of skeletal muscle of rat were used to be investigated for their chondrogenic and osteogenic potential as comparison to rat L6 myogenic cell line in vitro. Our results demonstrated that FDCs showed excellent chondrogenic potential and poor osteogenic potential, while myogenic cell line L6 displayed strong osteogenic potential and weak chondrogenic potential.
Additionally, by utilizing the FVB/N-TgN (TIE2-LacZ) 182Sato mouse, the Tie2+ cells did not contribute either chondroblasts or osteoblasts, which showed a controversial result to a pervious study in that Tie2-expressing progenitor cells differentiate through an endochondral pathway, contribute to every stage of the heterotopic endochondral formation[4]. We believed that it is possible due to differences in the genetic background of the mouse strains used in ours and their study.
However, in this regard, another study reported that mouse endothelial cells in their native in vivo do not participate in heterotopic ossification when exposed to BMP2, and Tie2+PDGFRα+Sca-1+ interstitial fibrogenic cells are the predominant BMP responsive mesenchymal progenitor of heterotopic skeletogenesis[29-30]. We agreed on first half of their conclusion and hold debatable opinion toward to last half of it. Our hypothesis was supported by the following three pieces of evidence. First one is that the heterotopic ossification of fibrodysplasia ossificans progressiva mainly happened in the skeletal muscle in which myogenic cells reside only. Second is that myogenic cells demonstrated much stronger osteogenic potential than fibrogenic cells, and fibrogenic cells displayed much strong chondrogenic potential than myogenic cells. The last one is the time frame of heterotopic ossification. Generally, the whole process of heterotopic ossification induced by AAV-BMP4 implantation in the skeletal muscle took about 14 days. It would be challenging for fibrogenic cells to differentiate into osteoblasts under the treatment of BMP4 in such a limited time period, while readily achieved for myogenic cells in the skeletal muscle (Figure 7).
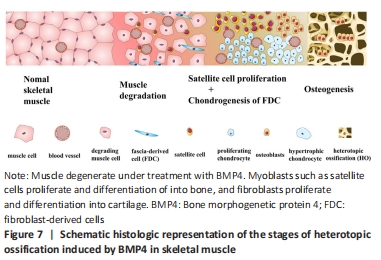
In summary, as shown in Figure 7, we concluded that fibrogenic cells residing in the skeletal muscle perimysium and endomysium might be the major contributing cell source for chondrocytes, while myogenic cells like the satellite cell may be the dominant progenitors for osteoblasts during the process of heterotopic ossification. The Tie2+ endothelial cells did not contribute the cell source for cartilage and bone phase. 中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
 #br#
#br#
文题释义:
异位骨化:指在正常情况下不具有骨化性质的组织中的骨形成,包括继发于肌肉、骨骼损伤后的异位骨化、神经源性异位骨化和进行性纤维发育不良性骨化等。其主要特点是在软组织中钙化骨迅速形成,于伤后3-12周形成异位骨组织,引起关节周围肿胀、疼痛及关节活动障碍等症状,甚至出现周围神经嵌压和压迫性溃疡。人骨形态发生蛋白4:作为骨形态发生蛋白家族成员之一,此蛋白在空间结构上与骨形态发生蛋白大体结构有一致性,只有在成熟区序列方面有其特异性。骨形态发生蛋白4在促进骨组织再生修复方面发挥着重要作用。此外,骨形态发生蛋白4也与诱导胚胎分化、指导神经干细胞分化、调节肿瘤生长侵袭以及一些心脑血管疾病密切相关。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
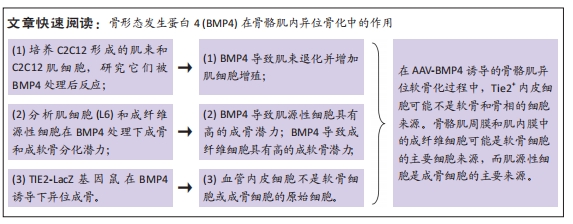
为研究细胞在骨肌中异位骨化过程中的作用。该实验在体外培养C2C12形成的肌束和C2C12肌母细胞,研究它们对骨形态发生蛋白4(BMP4)处理的反应。发现BMP4导致C2C12形成的肌束退化并增加C2C12细胞增殖。另外肌源性细胞系在BMP4处理下具有高的成骨潜力但较低成软骨潜力。最后该实验将AAV-BMP4吸附的明胶海绵移植到FVB/N-TgN(TIE2-LacZ)182Sato小鼠中。染色结果提示Tie2+血管内皮细胞不是软骨细胞或成骨细胞的原始细胞。该实验最后得出结论在AAV-BMP4诱导的骨骼肌异位软骨化过程中,Tie2+内皮细胞可能不是软骨和骨相的细胞来源。骨骼肌周膜和肌内膜中的成纤维细胞可能是软骨细胞的主要细胞来源,而肌源性细胞是成骨细胞的主要来源。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||