中国组织工程研究 ›› 2023, Vol. 27 ›› Issue (15): 2412-2419.doi: 10.12307/2023.609
• 干细胞综述 stem cell review • 上一篇 下一篇
转化生长因子β诱导骨髓间充质干细胞分化为半月板纤维软骨细胞
杨 均,李 澎
- 大连医科大学附属第二医院关节与运动医学科,辽宁省大连市 116023
-
收稿日期:2022-06-24接受日期:2022-07-27出版日期:2023-05-28发布日期:2022-10-18 -
通讯作者:李澎,博士,主任医师,大连医科大学附属第二医院关节与运动医学科,辽宁省大连市 116023 -
作者简介:杨均,男,1997年生,贵州省贵阳市人,汉族,大连医科大学在读硕士,主要从事骨关节外科方面的研究。 -
基金资助:大医二院-中科院大连化物所“个体化诊疗协同创新中心”联合基金项目(UF-ZD-202013),项目负责人:李澎;大连医科大学附属第二医院临床能力提升“1+X”计划交叉学科创新项目(2022JCXKYB18),项目负责人:李澎
Differentiation of bone marrow mesenchymal stem cells into meniscus fibrochondrocytes induced by transforming growth factor beta
Yang Jun, Li Peng
- Department of Joint and Sports Medicine, The Second Hospital of Dalian Medical University, Dalian 116023, Liaoning Province, China
-
Received:2022-06-24Accepted:2022-07-27Online:2023-05-28Published:2022-10-18 -
Contact:Li Peng, MD, Chief physician, Department of Joint and Sports Medicine, The Second Hospital of Dalian Medical University, Dalian 116023, Liaoning Province, China -
About author:Yang Jun, Master candidate, Department of Joint and Sports Medicine, The Second Hospital of Dalian Medical University, Dalian 116023, Liaoning Province, China -
Supported by:United Fund of the Second Hospital of Dalian Medical University and Dalian Institute of Chemical Physics, Chinese Academy of Sciences, No. UF-ZD-202013; “1+X”Program for Clinical Competency enhancement–Interdisciplinary Innovation Project, The Second Hospital of Dalian Medical University, No. 2022JCXKYB18 (to LP)
摘要:
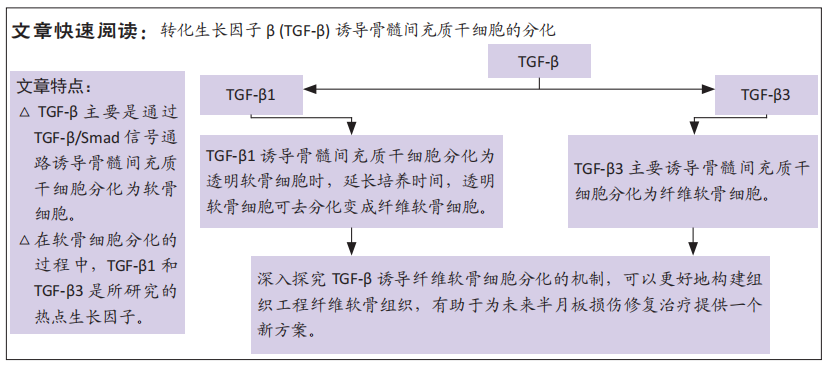
文题释义:
转化生长因子β:一种刺激骨髓间充质干细胞发育和分化的多肽生长因子,在诱导骨髓间充质干细胞分化为半月板纤维软骨细胞的过程中发挥多种生物学活性,例如参与到细胞迁移聚集、生长增殖和分化等多个过程。
骨髓间充质干细胞:一种来源于骨髓的成体干细胞,具有强大的增殖能力和多向分化潜能,在特定诱导条件下,可分化为成骨细胞、软骨细胞、脂肪细胞、肝细胞和神经细胞等。
背景:骨髓间充质干细胞可被诱导分化为纤维软骨细胞作为组织工程中的种子细胞,而细胞因子转化生长因子β(transforming growth factor beta,TGF-β1)和TGF-β3在诱导分化中的作用,目前仍是研究热点。
目的:综述TGF-β1和TGF-β3诱导骨髓间充质干细胞分化为软骨细胞的相关文献,为进一步指导TGF-β诱导骨髓间充质干细胞分化为纤维软骨细胞提供理论依据,同时为今后的临床上半月板损伤和退化的治疗提供新策略。
方法:计算机检索PubMed、中国知网和万方医学网数据库1990年1月至2022年3月收录的文献。英文数据库检索词:“Transforming Growth Factor beta 1,Transforming Growth Factor beta 3,Bone Marrow Mesenchymal Stem Cells,meniscus,fibrochondrocyte”,中文数据库检索词:“TGF-β1,TGF-β3、骨髓间充质干细胞、半月板、纤维软骨细胞”,根据筛选标准,最终纳入78篇文献进行综述。
结果与结论:①TGF-β主要分为TGF-β1、TGF-β2和TGF-β3共3种亚型,其中研究较多的细胞因子是TGF-β1和TGF-β3。②TGF-β主要通过TGF-β/Smad信号通路激活细胞核内的SOX9转录因子进而诱导骨髓间充质干细胞分化为软骨细胞。③TGF-β1可诱导骨髓间充质干细胞向透明软骨细胞分化,伴随培养时间的延长,透明软骨细胞可发生去分化变成纤维软骨细胞;④TGF-β3主要诱导骨髓间充质干细胞向纤维软骨细胞分化,同时促进Ⅰ型胶原和蛋白聚糖合成分泌。⑤目前,关于半月板损伤和退化的治疗,临床上采用的治疗方案均不理想,组织工程的出现为其治疗提供了新的策略,具体方法是将诱导因子和骨髓间充质干细胞植入到半月板损伤部位,可以起到恢复半月板功能及再生纤维软骨组织的作用,但是目前实验尚处于体外和动物模型阶段,其在临床上用于半月板受损的治疗和可用性仍需要后续大量的临床研究去研究探索。
https://orcid.org/0000-0001-9645-1997 (杨均);https://orcid.org/0000-0001-6859-5694 (李澎)
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
中图分类号:
引用本文
杨 均, 李 澎. 转化生长因子β诱导骨髓间充质干细胞分化为半月板纤维软骨细胞[J]. 中国组织工程研究, 2023, 27(15): 2412-2419.
Yang Jun, Li Peng. Differentiation of bone marrow mesenchymal stem cells into meniscus fibrochondrocytes induced by transforming growth factor beta[J]. Chinese Journal of Tissue Engineering Research, 2023, 27(15): 2412-2419.
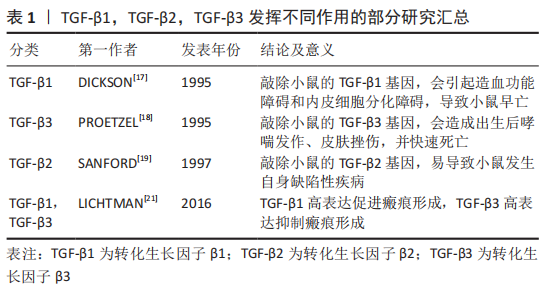
基因敲除的相关研究显示,在小鼠体内敲除TGF-β1基因可诱导胚胎发育过程中的造血功能障碍和内皮细胞分化障碍,导致小鼠早亡[17];在小鼠模型中敲除TGF-β3基因会导致小鼠严重腭裂、出生后哮喘发作、皮肤挫伤,并在24 h内快速死亡[18];在TGF-β2基因敲除的小鼠模型中,会发生小鼠在胚胎期易死亡且易发生自身缺陷性疾病[19]。另外,TGF-β3诱导分化的软骨细胞合成分泌胶原和蛋白聚糖的能力强于TGF-β1诱导分化的细胞[20]。TGF-β1和TGF-β3在创面愈合和瘢痕形成中也发挥着不同的作用,其中高表达TGF-β1可促进瘢痕形成,而高表达TGF-β3则具有抗纤维化、抑制瘢痕形成和促进组织细胞再生的效果[21]。
TGF-β在细胞外是处于一种非活性待激活状态,是由非共价键连接前体TGF-β和前体相关蛋白所组成,可在蛋白酶体催化、增强局部环境酸性、离子强度发生改变等条件下被激活。激活后TGF-β可与细胞膜表面的TGF-β受体结合,并进一步磷酸化Smad信号通路,促使细胞发挥各种生物学作用。前体相关蛋白在维持TGF-β的非活性状态中起到重要作用,只有前体TGF-β和前体相关蛋白分离后TGF-β才能发挥其生物学作用。
2.2 TGF-β受体的相关研究 TGF-β受体在TGF-β/Smad信号通路中的细胞膜上起着重要作用,TGF-β配体和TGF-β受体结合可将信号从细胞外传递到细胞内。在人体多种组织细胞膜上有着TGF-βⅠ型受体(transforming growth factorβ receptor 1,TβR-Ⅰ)、TGF-βⅡ型受体(transforming growth factorβ receptor 2,TβR-Ⅱ)和TGF-βⅢ型受体(transforming growth factorβ receptor 3,TβR-Ⅲ)。TGF-βⅠ型和TGF-βⅡ型受体属于糖蛋白,都为单次跨膜的丝氨酸/苏氨酸蛋白激酶受体,与TGF-β配体结合后可将相关信号传递到细胞内。在3种TGF-β亚型中,TGF-β1具有与TGF-βⅡ型受体最强的亲和力[22]。TGF-βⅢ受体可分为结合型和游离型两种类型,结合型受体具有增加TGF-β与 TGF-βⅠ型受体结合的能力,而游离型受体具有抑制TGF-β与TGF-βⅠ型受体结合的能力[23]。其中,TGF-βⅠ型受体又称为激活素受体样激酶(activin receptor-like kinase,ALKs),可分为ALK1-ALK7这7种表现形式[24]。在TGF-β诱导骨髓间充质干细胞分化为软骨细胞中,TGF-β主要刺激TGF-βⅠ型受体中的ALK5磷酸化,进而起到传递信号并可诱导细胞分化。
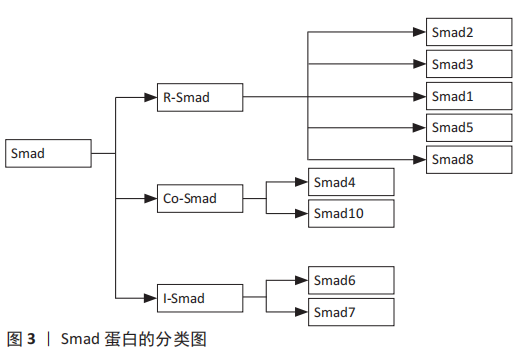
2.3 Smad蛋白的相关研究 Smad蛋白分布于细胞质中,在TGF-β/Smad信号通路中发挥着重要作用,它可将信号从细胞膜传递到细胞核中。Smad蛋白又是相对分子质量为42 000-60 000的蛋白分子,主要由2个保守的结构域构成,即N端的Mad同源性1(MH1)结构域和C端的Mad同源性2(MH2)结构域,其中,MH1介导DNA的连接,MH2可上调转录因子活性[25]。此外,Smad蛋白还能调节控制其下游靶基因转录的过程,根据Smad蛋白组成结构和生理功能的不同,Smads蛋白主要划分为3种类型:①受体激活型Smads (R-Smads):Smad2,Smad3,Smad1,Smad5和Smad8,其中TGF-β调控Smad2和Smad3发挥信号传导作用,而骨形态发生蛋白则会调控Smad1,Smad5和Smad8介导信号传导;②共同介导型Smad(Co-Smad):Smad4和Smad10。Smad4是R-Smad传导信号的下游分子,磷酸化的Smad2/3必须先与Smad4结合成三聚体复合物才能进入到细胞核中,并与DNA序列上的Smad结合元件结合从而调节转录活动;③抑制型Smads(I-smads):Smad6,Smad7。Smad7可抑制Smad3的磷酸化,并干扰R-Smad与TGF-βI受体或Smad4的结合,进而对TGF-β/Smad信号通路产生抑制作用[26]。
TGF-β诱导骨髓间充质干细胞向软骨细胞分化的过程中,磷酸化Smad2,Smad3,Smad4组成三聚体复合体,进入到细胞核与DNA序列上的Smad结合元件结合,可调节其靶基因的表达。Smad2的MH1区域比Smad3的MH1多了2个氨基酸片段,导致其缺乏与DNA结合的活性,Smad2,3,4三聚体复合体主要依靠Smad3和Smad4的Mad同源性1(MH1)结构域与DNA结合[27]。
此外,Smad7可激活E3泛素连接酶即Smad泛素化调节因子1(SMURF1)和Smad泛素化调节因子2(SMURF2)表达,其与R-Smad蛋白结合可使R-Smad蛋白降解,从而拮抗TGF-β/Smad通路[28-29]。Smads蛋白是一种细胞内信号转导分子,可将相应的信号从细胞内传递到细胞核,TGF-β的各种调节作用就是通过这一途径实现的。抑制Smad2/3的磷酸化会导致细胞失去合成分泌胶原蛋白的能力,进而导致软骨细胞分化停止,说明Smads2/3在骨髓间充质干细胞分化为软骨细胞中发挥着重要作用。
Smad蛋白的具体分类见图3。
2.4 SOX9转录因子的相关研究 在TGF-β/Smad信号通路中,Y染色体性别决定区(sex-determing region of Y chromosome,SRY)-盒转录因子9(SRY-box transcription factor 9,Sox9)位于细胞核上,可被激活调控间充质干细胞向软骨细胞系分化的关键转录因子。在TGF-β通过smad信号通路诱导骨髓间充质干细胞向纤维软骨细胞分化的过程中,首先在细胞核中激活SOX9,进而促进Ⅰ型胶原蛋白和蛋白聚糖的合成分泌。SOX9在间充质干细胞的迁移、聚集和启动诱导间充质干细胞向着纤维软骨细胞分化的过程中有着重要作用,SOX9还被认为是软骨细胞形成早期的标志物,该阶段细胞具备了合成分泌胶原蛋白和蛋白聚糖的能力[30]。SOX9与Y染色体上SRY基因高度同源,是一种与软骨形成相关的重要转录因子,SOX9基因突变会导致身高发育不良、鼻梁凹陷、四肢短小及骨骼异常等临床表现[31]。将表达SOX9基因的质粒转染至间充质干细胞可获得软骨细胞表型,而敲除SOX9基因的间充质干细胞细胞则不能分化成软骨细胞[32]。此外,SOX9因子上有着多个微小RNA(microRNA)的结合位点,在软骨细胞分化过程中miR-495可与SOX9结合起到抑制分化的作用[33]。实验中应用基因转染技术,过表达miR-495可抑制SOX9活性,并抑制间充质干细胞分化为软骨细胞,进而抑制细胞外基质的合成分泌。miR-145、miR-194 和 miR-199a 可下调SOX9转录活性并起到抑制软骨形成的作用[34-36]。
SOX5和SOX6基因在间充质干细胞向软骨细胞分化过程中也起着重要作用,SOX5,SOX6可与SOX9结合起到加强诱导分化的作用。在软骨细胞分化过程中,即使SOX9转录因子可正常表达,但SOX5和SOX6基因失效的话,将导致间充质干细胞分化为软骨细胞失败[37]。在TGF-β诱导骨髓间质干细胞分化为软骨细胞的过程中,SOX9可以通过抑制β-连环蛋白的活性来促进其向软骨细胞分化。SOX9抑制β-连环蛋白活性的机制是通过与β-连环蛋白结合[38],抑制β-连环蛋白的活性,还可以降解β-连环蛋白,进而促进骨髓间充质干细胞向软骨细胞分化。
2.5 TGF-β通过Smad信号通路诱导骨髓间充质干细胞分化为软骨细胞 具体机制见图4。
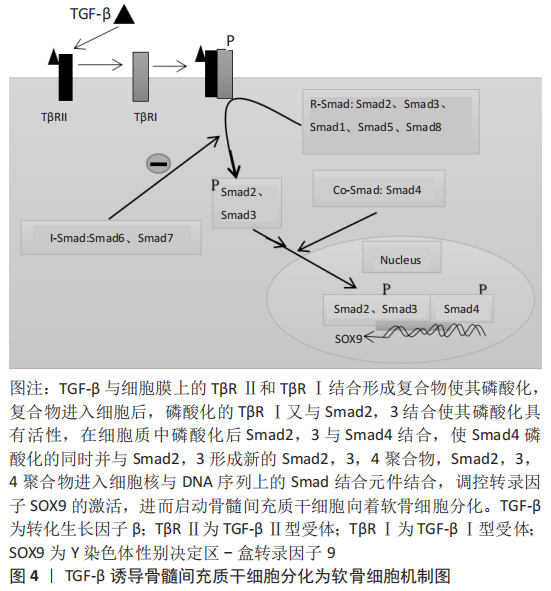
TGF-β具有促进骨髓间充质干细胞迁移聚积、增殖和分化的作用,在维持软骨细胞的表型中也起到重要作用,并可调节细胞外基质的合成分泌。TGF-β诱导骨髓间充质干细胞分化为软骨细胞的机制是,TGF-β在细胞外被激活,激活后的TGF-β与TβRⅡ型、TβRⅠ型两种受体结合使其磷酸化,并在细胞膜表面形成三聚体复合物[39]。然后,细胞膜上的网格蛋白经过内吞作用将复合物从细胞膜外输送到细胞膜内。复合物进入细胞后,活化的Ⅰ型受体(ALK5)与Smad2,3结合,并使Smad2,3磷酸化具有活性。随后,磷酸化的Smad2、3从复合物中分离出来并和Samd4结合[40],使其Samd4磷酸化的同时并组成具有与DNA结合能力的Smad2,3,4三聚体复合物。Smad2,3,4三聚体复合物进入细胞核后直接与DNA序列上的smad结合元件结合,调控SOX9转录因子的激活[41],促进Ⅰ胶原和蛋白聚糖的合成,并诱导骨髓间充质干细胞分化为软骨细胞。SOX5和SOX6在间充质干细胞分化过程中与SOX9结合形成转录复合体,可起到加强诱导分化的作用[42-43]。Samd与DNA结合活性取决于Smad2,3,4的mad同源性(MH1)结构域。
此外,Smad2和Smad3的MH2结构域已展现出可与组蛋白乙酰转移酶活性的共激活因子CBP/p300结合并发挥促进作
用[44]。因此,激活CBP/p300因子可增强SOX9转录活性,进而可促进间充质干细胞分化为软骨细胞。此外,SOX9是间充质干细胞分化为软骨细胞的关键转录因子之一。SOX9的缺失将导致间充质干细胞不能分化为软骨细胞,从而导致细胞不能合成分泌细胞外基质[45]。SOX9的过表达不仅可以促进间充质干细胞的迁移聚集,还可以抑制软骨细胞转变为软骨肥大细胞表型,进而抑制基质金属蛋白酶13、碱性磷酸酶和X型胶原的合成分泌。
2.5.1 TGF-β1对骨髓间充质干细胞分化为软骨细胞的作用 TGF-β1是一种可参与调控细胞的多种生物学活动的多肽生长因子,具有调控细胞生长增殖、刺激细胞分化,降低免疫反应等作用。另外,TGF-β1还可以刺激间充质干细胞的迁移和刺激细胞外基质的产生[46]。TGF-β1不仅可促进软骨细胞的生长增殖、细胞外基质的合成分泌,还具有促进组织金属蛋白酶抑制剂的合成分泌进而减少软骨基质的降解[47]。在关节炎症的软骨修复中,TGF-β1还可起到减轻炎症的作用,有利于软骨组织更好地进行修复[48]。文章将TGF-β1诱导骨髓间充质干细胞分化为软骨细胞的部分研究汇总[52-56],见表2。
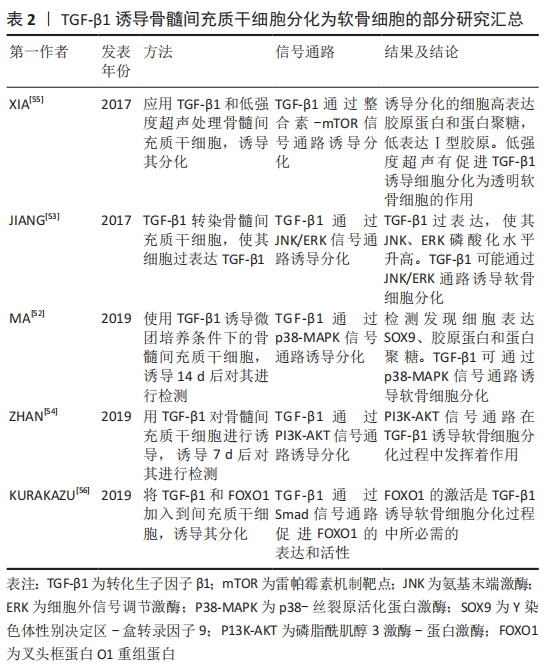
TGF-β1通过与细胞膜表面的受体结合诱导骨髓间充质干细胞分化为软骨细胞,且能促进诱导后的细胞合成分泌细胞外基质胶原蛋白和蛋白聚糖,抑制Ⅰ型胶原的合成。LUBOS等[49]研究表明,在含有间充质干细胞的培养基中添加TGF-β1,将导致Ⅰ型胶原基因表达的下调以及透明软骨典型的胶原蛋白和蛋白聚糖基因表达的上调。在TGF-β1诱导软骨细胞分化过程中,将骨髓间充质干细胞种植在支架上,诱导细胞分化的效果比没有支架的要明显。LIAO等[50]研究发现,将TGF-β1细胞因子添加到接种有骨髓间充质干细胞的含有软骨细胞外基质的静电纺丝微纤维支架中,培养21 d后,检测发现胶原蛋白和蛋白聚糖的mRNA上调,Ⅰ型胶原mRNA下调,合成分泌的基质比没有将细胞种植在支架上的多。
考虑到外源性TGF-β1细胞因子的价格较贵,有人提出将TGF-β1转染入骨髓间充质干细胞,骨髓间充质干细胞在TGF-β1基因过表达的情况下,可稳定促进诱导分化。陈祁青等[51]研究证实,TGF-β1基因经慢病毒载体转染骨髓间充质干细胞培养14 d后,分别行RT-PCR法检测TGF-β1、胶原蛋白的mRNA的表达,免疫细胞化学染色、甲苯胺蓝染色,结果表明TGF-β1基因转染入骨髓间充质干细胞可稳定表达、胶原蛋白的mRNA表达增加、甲苯胺蓝染色和免疫细胞化学染色均为阳性,表明TGF-β1转染入骨髓间充质干细胞可促进其向软骨细胞分化。
TGF-β1诱导软骨细胞分化过程中,不仅依靠主要的Smad信号通路,还可通过非Smad信号通路诱导其分化。MA等[52]通过研究发现,TGF-β1在诱导软骨细胞分化过程中,可通过上调p38-丝裂原活化蛋白激酶(mitogen-activated protein kinases,MAPK)信号通路,进而促进SOX9、胶原蛋白和蛋白聚糖的表达。JIANG等[53]通过实验发现,在软骨细胞分化过程中,过表达TGF-β1可增加氨基末端激酶(JNK)、细胞外信号调节激酶(ERK)磷酸化水平,促进SOX9、胶原蛋白及蛋白聚糖的表达,敲除TGF-β1将导致JNK、ERK磷酸化水平降低和分化受阻,说明TGF-β1可通过JNK/ERK信号通路诱导软骨细胞分化。ZHAN等[54]
通过实验表明,TGF-β1可通过磷脂酰肌醇3激酶-蛋白激酶(P13K-AKT)信号通路诱导骨髓间充质干细胞分化软骨细胞。Xia等[55]研究表明,在TGF-β1通过整合素-机制靶点雷帕霉素(mTOR)信号通路诱导骨髓间充质干细胞分化为软骨细胞的过程中,低强度超声加强了TGF-β1的诱导效应,表现为胶原蛋白、蛋白聚糖和SOX9基因表达增加,Ⅰ型胶原表达减少。
KURAKAZU等[56]研究表明,TGFβ1通过SMAD信号通路增加叉头框蛋白O1(FOXO1)重组蛋白(recombinant fork head box protein O1,FOXO1)的表达和活性,进而诱导间充质干细胞分化为软骨细胞,并且 FOXO1是胶原蛋白和蛋白聚糖表达所需要的。FOXO1还可介导p21的表达来促进G0/G1期的细胞周期停滞,进而促进分化。在TGF-β1诱导骨髓间充质干细胞向软骨细胞分化过程中,细胞因子的浓度也是一个重要因素,浓度过高会抑制其分化,浓度过低诱导分化效果则不足。邓进等[57]研究表明,使用质量浓度为1,5,10,15和20 μg/L的TGF-β1诱导骨髓间充质干细胞向软骨细胞分化,14 d后对其进行检测,发现质量浓度为10 μg/L的TGF-β1诱导分化效果最强。俞猛等[58]通过实验证实,TGF-β1质量浓度为10 ng/mL时,诱导骨髓间充质干细胞分化为软骨细胞的效果最佳。
TGF-β1细胞因子还可与其他物质协同促进软骨细胞分化。高子笼等[59]使用质量浓度为10 μg/L的TGF-β1和补骨脂素诱导骨髓间充质干细胞,结果表明单独应用TGF-β1组胶原蛋白表达阳性、SOX9和胶原蛋白的mRNA表达增加,但联合使用TGF-β1和补骨脂素组,其SOX9和胶原蛋白的mRNA的表达量最明显,说明TGF-β1具有诱导软骨细胞分化的特性,补骨脂素可增加TGF-β1诱导分化的能力。此外,当TGF-β1诱导骨髓间充质干细胞分化为透明软骨细胞时,伴随培养时间的延长,透明软骨细胞有向着纤维软骨细胞去分化的倾向,表现为Ⅰ型胶原合成增多和蛋白聚糖合成减少[60]。因此,在TGF-β1诱导骨髓间充质干细胞分化的基础上,诱导培养时间也是一个重要因素。
总而言之,TGF-β1主要通过Smad信号通路,同时也通过非Smad信号通路诱导骨髓间充质干细胞分化为软骨细胞,上调SOX9转录因子、促进胶原蛋白和蛋白聚糖表达,并抑制Ⅰ型胶原表达。因此推断,TGF-β1具有诱导骨髓间充质干细胞分化为透明软骨细胞的作用,但是随着培养时间的延长,透明软骨细胞可发生去分化变成纤维软骨细胞。
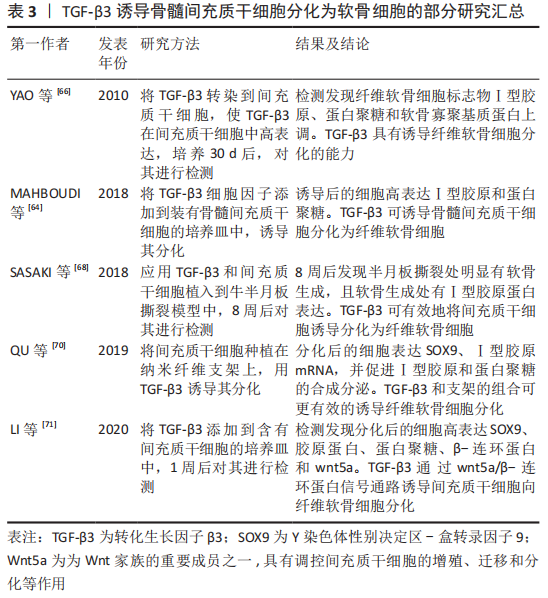
2.5.2 TGF-β3对骨髓间充质干细胞分化为软骨细胞所起作用 TGF-β3已被确定为骨髓间充质干细胞发育和分化的有效刺激因子,在调控骨髓间充质干细胞分化为软骨细胞过程中发挥多种生物学活动,并参与到细胞迁移聚集、生长增殖和分化等多个过程。此外,TGF-β3还可刺激软骨细胞增殖,促进Ⅰ型胶原和蛋白聚糖等细胞外基质的合成分泌[61-62]。
TGF-β3作为纤维软骨组织形成的重要细胞因子,在骨髓间充质干细胞迁移和纤维软骨细胞分化,增强半月板组织缺损修复的过程中起到非常重要作用[63],是一种诱导纤维软骨细胞分化的关键细胞因子。TGF-β3可促进纤维软骨细胞分化,并促进其基质Ⅰ型胶原和蛋白聚糖的合成分泌。MAHBOUDI等[64]通过实验发现,TGF-β3可诱导骨髓间充质干细胞分化为纤维软骨细胞,并促进SOX9、Ⅰ型胶原和蛋白聚糖的合成分泌,其中Ⅰ型胶原的合成在21 d内,呈时间依赖性增加。LIANG等[65]研究表明,TGF-β3可促进种植于半月板来源的脱细胞基质中的间充质干细胞合成软骨基质,并上调蛋白聚糖、Ⅰ胶原蛋白表达,说明TGF-β3具有诱导间充质干细胞向着纤维软骨细胞分化的可行性。将TGF-β3转染入骨髓间充质干细胞,也是其中一种促进分化的方法,且比外源性添加细胞因子更加稳定有效。
YAO等[66]研究表明,TGF-β3基因经腺病毒转染间充质干细胞,培养30 d后,检测发现软骨细胞标志物Ⅰ型胶原、蛋白聚糖和软骨寡聚基质蛋白上调,Ⅰ型胶原显著升高,说明TGF-β3转染入骨髓间充质干细胞可稳定向着纤维软骨细胞分化。将骨髓间充质干细胞种植在支架上,添加TGF-β3细胞因子可充分诱导其分化,另外将TGF-β3和骨髓间充质干细胞及支架同时植入动物半月板缺损模型中,可明显促进半月板损伤处软骨的恢复。
MAHMOUDI等[67]研究表明,负载有TGF-β3的海藻酸钠凝胶可诱导间充质干细胞分化为软骨细胞,且促进软骨标志物胶原蛋白、蛋白聚糖高表达。SASAKI等[68]将间充质干细胞和质量浓度为10 ng/mL的水溶性TGF-β3添加到牛半月板撕裂模型中,8周后在半月板撕裂部位强表达Ⅰ型胶原,软骨缺损处有明显恢复。
刘莹松等[69]将TGF-β3和海藻酸钠水凝胶复合骨髓间充质干细胞植入兔膝关节软骨缺损处,检测发现胶原蛋白、SOX9和蛋白聚糖的mRNA显著上调,表明TGF-β3和海藻酸钠复合骨髓间充质干细胞可促进膝关节软骨缺损的修复,在组织缺损处发现有再生组织。TGF-β3除通过主要的Smad通路诱导软骨细胞分化,还可通过其他通路诱导分化。QU等[70]研究发现,TGF-β3可增加Smad2、Smad3磷酸化,在诱导间充质干细胞分化为纤维软骨细胞的同时,高表达SOX9、Ⅰ型胶原的mRNA,并促进Ⅰ型胶原和蛋白聚糖的合成分泌,表明TGF-β3可通过Smad通路诱导纤维软骨细胞分化。
LI等[71]为探究TGF-β3在间充质干细胞分化为软骨细胞中的作用,用质量浓度为100 μg/L的TGF-β3诱导间充质干细胞,诱导1周后检测发现,SOX9、β-连环蛋白、蛋白聚糖及wnt5a呈高表达,说明TGF-β3可通过wnt5a/β-连环蛋白信号通路诱导间充质干细胞分化为软骨细胞。TGF-β3联合其他物理刺激或细胞因子可比单独使用TGF-β3诱导软骨细胞分化效果有效。GUHA等[72]分别或同时应用低强度的超声和TGF-β3诱导骨髓间充质干细胞分化为软骨细胞,结果表明,TGF-β3和低强度的超声联合诱导组的细胞外基质胶原蛋白和蛋白聚糖表达最多,且miR-145在间充质干细胞软骨形成早期被显著下调,说明低强度超声可协同促进TGF-β3的诱导分化作用,miR-145的表达可定位TGF-β3促分化过程的不同阶段。
HUANG等[73]研究表明,TGF-β3可诱导间充质干细胞分化为软骨细胞,成纤维细胞生长因子18和TGF-β3联合应用亦可诱导软骨细胞分化,检测发现成纤维细胞生长因子18和TGF-β3同时应用时胶原蛋白、蛋白聚糖及SOX9的mRNA表达量显著高于单独使用TGF-β3,说明TGF-β3和成纤维细胞生长因子18协同促进软骨细胞分化。在使用TGF-β3诱导软骨细胞分化时,在诱导时间控制上也是一个重要因素。刘登榜等[74]研究提示,在体外使用10 μg/L TGF-β3诱导大鼠的骨髓间充质干细胞分化为软骨细胞时,在培养7,14 d时胶原蛋白免疫组织化学染色结果无明显差异,说明TGF-β3可较快地诱导骨髓间充质干细胞分化为软骨细胞。
文章将TGF-β3诱导骨髓间充质干细胞分化为软骨细胞的部分研究结果汇总,见表3。
总之,TGF-β3是诱导纤维软骨细胞分化的重要细胞因子,通过上调SOX9转录因子的表达促进蛋白聚糖、Ⅰ型胶原的合成分泌。TGF-β3在诱导骨髓间充质干细胞分化为纤维软骨细胞的过程中,多条信号通路发挥着不同的作用,其中关于TGF-β3/Smad信号通路理论大家已经达成共识并被认可。
| [1] GEE SM, TENNENT DJ, CAMERON KL, et al. The burden of meniscus injury in young and physically active populations. Clin Sports Med. 2020;39(1):13-27. [2] CHUNG KS, HA JK, KIM YS, et aL. National trends of meniscectomy and meniscus repair in Korea. J Korean Med Sci. 2019;34(32):e206. [3] BAHCECIOGLU G, BILGEN B, HASIRCI N, et aL. Anatomical meniscus construct with zone specific biochemical composition and structural organization. Biomaterials. 2019;218:119361. [4] LOMBARDO MDM, MANGIAVINI L, PERETTI GM. Biomaterials and meniscal lesions: current concepts and future perspective. Pharmaceutics. 2021;13(11): 1886. [5] OLDERSHAW RA. Cell sources for the regeneration of articular cartilage: the past, the horizon and the future. Int J Exp Pathol. 2012 93(6):389-400. [6] KWON H, BROWN WE, LEE CA, et aL. Surgical and tissue engineering strategies for articular cartilage and meniscus repair. Nat Rev Rheumatol. 2019;15(9):550-570. [7] HUANG K, LI Q, LI Y, et aL. Cartilage tissue regeneration: the roles of cells, stimulating factors and scaffolds. Curr Stem Cell Res Ther. 2018;13(7):547-567. [8] 肖丹,江东,余家阔.半月板干细胞在半月板损伤修复中的研究现状[J].中国矫形外科杂志,2018,26(22):2083-2087. [9] ZHONG G, YAO J, HUANG X, et aL. Injectable ECM hydrogel for delivery of BMSCs enabled full-thickness meniscus repair in an orthotopic rat model. Bioact Mater. 2020;5(4):871-879. [10] HIDALGO PEREA S, LYONS LP, NISHIMUTA JF, et aL. Evaluation of culture conditions for in vitro meniscus repair model systems using bone marrow-derived mesenchymal stem cells. Connect Tissue Res. 2020;61(3-4):322-337. [11] NOBLE BS, DEAN V, LOVERIDGE N, et aL. Dextran sulfate promotes the rapid aggregation of porcine bone-marrow stromal cells. Bone. 1995;17(4):375-382. [12] MACKAY AM, BECK SC, MURPHY JM, et aL. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 1998;4(4): 415-428. [13] FUKUMOTO T, SPERLING JW, SANYAL A, et al. Combined effects of insulin-like growth factor-1 and transforming growth factor-beta1 on periosteal mesenchymal cells during chondrogenesis in vitro. Osteoarthritis Cartilage. 2003;11(1):55-64. [14] RE’EM T, KAMINER-ISRAELI Y, RUVINOV E, et al. Chondrogenesis of hMSC in affinity-bound TGF-beta scaffolds. Biomaterials. 2012;33(3):751-761. [15] DE LARCO JE, TODARO GJ.Growth factors from murine sarcoma virus transformed cells.Proc Natl Aead Sei U S A. 1978;8:4001-4005. [16] PELTON RW, SAXENA B, JONES M, et al. Immunohistochemical localization of TGF beta 1, TGF beta 2, and TGF beta 3 in the mouse embryo: expression patterns suggest multiple roles during embryonic development. J Cell Biol. 1991; 115(4):1091-105. [17] DICKSON MC, MARTIN JS, COUSINS FM, et al. Defective haematopoiesis and vasculogenesis in transforming growth factor-beta 1 knock out mice. Development. 1995;121(6):1845-1854. [18] PROETZEL G, PAWLOWSKI SA, WILES MV, et al. Transforming growth factor-beta 3 is required for secondary palate fusion. Nat Genet. 1995;1(4):409-414. [19] SANFORD LP, ORMSBY I, GITTENBERGER-DE GROOT AC, et al. TGF-β2 knockout mice have multiple developmental defects that are non-overlapping with other TGF B knockout phenotypes. Development. 1997;124:2659-2670. [20] BARRY F, BOYNTON RE, LIU B, et al. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiationdependent gene expression of matrix components. Exp Cell Res. 2001;268(2):189-200. [21] LICHTMAN MK, OTERO-VINAS M, FALANGA V. Transforming growth factor beta (TGF-β) isoforms in wound healing and fibrosis. Wound Repair Regen. 2016; 24(2):215-222. [22] 魏丽,刘萌萌,屈彩霞.TGF-β_1与α-SMA在小鼠肺纤维化模型中的表达及其相互关系[J].中国现代医生,2016,54(22):31-33,169. [23] BLOBE GC, SCHIEMANN WP, PEPIN MC, et al. Functional roles for the cytoplasmic domain of the type III transforming growth factor beta receptor in regulating transforming growth factor beta signaling. J Biol Chem. 2001;276(27):24627-24637. [24] NICKEL J, TEN DIJKE P, MUELLER TD. TGF-β family co-receptor function and signaling. Acta Biochim Biophys Sin (Shanghai). 2018;50(1):12-36. [25] MAKKAR P, METPALLY RP, SANGADALA S, et aL. Modeling and analysis of MH1 domain of Smads and their interaction with promoter DNA sequence motif. J Mol Graph Model. 2009;27(7):803-812. [26] SONG B, ESTRADA KD, LYONS KM. Smad signaling in skeletal development and regeneration. Cytokine Growth Factor Rev. 2009;20(5-6):379-388. [27] SHI Y, WANG YF, JAYARAMAN L, et al. Crystal structure of a Smad MH1 domain bound to DNA: insights on DNA binding in TGF-beta signaling. Cell. 1998;94(5):585-594. [28] HANYU A, ISHIDOU Y, EBISAWA T, et al. The N domain of Smad7 is essential for specific inhibition of transforming growth factor-beta signaling. J Cell Biol. 2001;155(6):1017-1027. [29] KAVSAK P, RASMUSSEN RK, CAUSING CG, et al. Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets the TGF beta receptor for degradation. Mol Cell. 2000;6(6):1365-1375. [30] LIU CF, ANGELOZZI M, HASEEB A, et aL. SOX9 is dispensable for the initiation of epigenetic remodeling and the activation of marker genes at the onset of chondrogenesis. Development. 2018;145(14):dev164459. [31] CROFT B, OHNESORG T, HEWITT J, et al, Human sex reversal Is caused by duplication or deletion of core enhancers upstream of Sox9. Nat Commun. 2018; 9(1):5319. [32] BI W, HUANG W, WHITWORTH DJ, et al. Haploinsufficiency of Sox9 results in defective cartilage primordia and premature skeletal mineralization. Proc Natl Acad Sci U S A. 2001;98(12):6698-6703. [33] LEE S, YOON DS, PAIK S, et al. microRNA-495 inhibits chondrogenic differentiation in human mesenchymal stem cells by targeting Sox9. Stem Cells Dev. 2014;23(15): 1798-1808. [34] LIN EA, KONG L, BAI XH, et al. miR-199a, a bone morphogenic protein 2-responsive MicroRNA, regulates chondrogenesis via direct targeting to Smad1. J Biol Chem. 2009;284(17):11326-11335. [35] YANG B, GUO H, ZHANG Y, et al. MicroRNA-145 regulates chondrogenic differentiation of mesenchymal stem cells by targeting Sox9. PLoS One. 2011;6(7):e21679. [36] XU J, KANG Y, LIAO WM, et al. MiR-194 regulates chondrogenic differentiation of human adipose-derived stem cells by targeting Sox5. PLoS One. 2012;7(3):e31861. [37] LIU CF, LEFEBVRE V. The transcription factors SOX9 and SOX5/SOX6 cooperate genome- wide through super-enhancers to drive chondrogenesis. Nucleic Acids Res. 2015;43(17):8183-8203. [38] TAKEGAMI Y, OHKAWARA. B, ITO M, et al. R.facilitates differentiation of proliferating spondin chondrocytes into hypertrophic chondrocytes by enhancing Wnt/B-catenin signaling in endochondral ossification. Biochem Biophys Res Commu. 2016;473(1):255-264. [39] MASSAGUÉ J, BLAIN SW, LO RS. TGFbeta signaling in growth control, cancer, and heritable disorders. Cell. 2000;103(2):295-309. [40] MIYAZAWA K, SHINOZAKI M, HARA T, et al. Two major Smad pathways in TGF-β superfamily signalling. Genes Cells. 2002;7(12):1191-204. [41] AUGUSTYNIAK E, TRZECIAK T, RICHTER M, et al. The role of growth factors in stem cell-directed chondrogenesis: a real hope for damaged cartilage regeneration. Int Orthop. 2015;39(5):995-1003. [42] LEFEBVRE V, LI P, DE CROMBRUGGHE B. A new long form of Sox5 (L-Sox5), Sox6 and Sox9 are coexpressed in chondrogenesis and cooperatively activate the type II collagen gene. EMBO J. 1998;17(19):5718-5733. [43] LIU CF, VERONIQUE L. The transcription factors SOX9 and SOX5/SOX6 cooperate genome-wide through superenhancers to drive chondrogenesis. Nucleic Acids Res. 2015;43(17):8183-8203. [44] POUPONNOT C, JAYARAMAN L, MASSAGUE J. Physical and functional interaction of SMADs and p300/CBP. J Biol Chem. 1998;273(36):22865-22868. [45] LEFEBVRE V, DE CROMBRUGGHE B. Toward understanding SOX9 function in chondrocyte differentiation. Matrix Biol. 1998;16(9):529-540. [46] HAGMEIJER MH, KORPERSHOEK JV, CRISPIM JF, et al. The regenerative effect of different growth factors and platelet lysate on meniscus cells and mesenchymal stromal cells and proof of concept with a functionalized meniscus implant. J Tissue Eng Regen Med. 2021;15(7):648-659. [47] ZHU Y, GU J, ZHU T, et al. Crosstalk between Smad2/3 and specific isoforms of ERK in TGF-β1-induced TIMP-3 expression in rat chondrocytes. J Cell Mol Med. 2017;21(9):1781-1790. [48] WU T, CHEN Y, LIU W, et al. Ginsenoside Rb1/TGF-β1 loaded biodegradable silk fibroin-gelatin porous scaffolds for inflammation inhibition and cartilage regeneration. Mater Sci Eng C Mater Biol Appl. 2020;111:110757. [49] LUBOS D, PETR L, VOJTECH H, et al. Chondrogenic differentiation of human bone marrow and adipose tissue-derived mesenchymal stem cells. J Appl Biomed. 2007. doi: 10.32725/jab.2007.019. [50] LIAO J, GUO X, GRANDE-ALLEN KJ, et al. Bioactive polymer/extracellular matrix scaffolds fabricated with a flow perfusion bioreactor for cartilage tissue engineering. Biomaterials. 2010;31(34):8911-8920. [51] 陈祁青,金红婷,应俊,等.慢病毒介导TGF-β1基因诱导大鼠骨髓间充质干细胞成软骨细胞分化[J].中国矫形外科杂志,2015,23(7):637-643. [52] MA N, TENG X, ZHENG Q, et al. The regulatory mechanism of p38/MAPK in the chondrogenic differentiation from bone marrow mesenchymal stem cells. J Orthop Surg Res. 2019;14(1):434. [53] JIANG X, HUANG B, YANG H, et al. TGF-β1 is Involved in vitamin D-induced chondrogenic differentiation of bone marrow-derived mesenchymal stem cells by regulating the ERK/JNK pathway. Cell Physiol Biochem. 2017;42(6):2230-2241. [54] ZHAN X, CAI P, LEI D, et al. Comparative profiling of chondrogenic differentiation of mesenchymal stem cells (MSCs) driven by two different growth factors. Cell Biochem Funct. 2019;37(5):359-367. [55] XIA P, WANG X, QU Y, et al. TGF-β1-induced chondrogenesis of bone marrow mesenchymal stem cells is promoted by low-intensity pulsed ultrasound through the integrin-mTOR signaling pathway. Stem Cell Res Ther. 2017;8(1):281. [56] KURAKAZU I, AKASAKI Y, HAYASHIDA M, et al. FOXO1 transcription factor regulates chondrogenic differentiation through transforming growth factor β1 signaling. J Biol Chem. 2019;294(46):17555-17569. [57] 邓进,彭吾训,王蕾,等.骨髓间充质干细胞二维培养条件下向 软骨细胞诱导分化的实验研究[J].中国现代医学杂志,2008,18(16):2340-2343. [58] 俞猛,于方,付胜良.兔骨髓间充质干细胞体外培养定向诱导分化为软骨细胞 [J].中国组织工程研究与临床康复,2011,15(27):4951-4954. [59] 高子茏,李婷,吕政,等.补骨脂素联合转化生长因子β1诱导骨髓间充质干细胞向软骨细胞的分化[J].中国组织工程研究,2022,26(30):4884-4888. [60] CAMARERO-ESPINOSA S, ROTHEN-RUTISHAUSER B, FOSTER EJ, et al. Articular cartilage: from formation to tissue engineering. Biomater Sci. 2016;4(5):734-767. [61] ROGER Y, SYDOW S, BURMEISTERL, et al. Sustained release of TGF- β3 from polysaccharide nanoparticles induces chondrogenic differentiation of human mesenchymal stromal cells. Colloids Surf B Biointerfaces. 2020;189:110843. [62] JING H, GAO B, GAO M, et al. Restoring tracheal defects in a rabbit model with tissue engineered patches based on TGF-beta3-encapsulating electrospun poly (l-lactic acid-co-epsilon-caprolactone)/collagen scaffolds. Artif Cells Nanomed Biotechnol. 2018;46(sup1):985-995. [63] SZOJKA ARA, LYONS BD, MOORE CN, et al. Hypoxia and TGF-β3 synergistically mediate inner meniscus-like matrix formation by fibrochondrocytes. Tissue engineering Part A. 2019;25(5-6):446-456. [64] MAHBOUDI H, SOLEIMANI M, HANAEE-AHVAZ H, et al. New approach for differentiation of bone marrow mesenchymal stem cells toward chondrocyte cells with overexpression of microRNA-140. ASAIO J. 2018;64(5):662-672. [65] LIANG Y, IDREES E, SZOJKA ARA, et al. Chondrogenic differentiation of synovial fluid mesenchymal stem cells on human meniscus-derived decellularized matrix requires exogenous growth factors. Acta Biomater. 2018;80:131-143. [66] YAO Y, ZHANG F, ZHOU R, et al. Effects of combinational adenoviral vector-mediated TGF beta 3 transgene and shRNA silencing type I collagen on articular chondrogenesis of synovium-derived mesenchymal stem cells. Biotechnol Bioeng. 2010;106(5):818-828. [67] MAHMOUDI Z, MOHAMMADNEJAD J, RAZAVI BAZAZ S, et al. Promoted chondrogenesis of hMCSs with controlled release of TGF-β3 via microfluidics synthesized alginate nanogels. Carbohydr Polym. 2020;229:115551. [68] SASAKI H, ROTHRAUFF BB, ALEXANDER PG, et al. In vitro repair of meniscal radial tear with hydrogels seeded with adipose stem cells and TGF-β3. Am J Sports Med. 2018;46(10):2402-2413. [69] 刘莹松,郭晓鹏,魏明珠.转化生长因子β3复合海藻酸钠水凝胶修复膝关节软骨缺损[J].中国组织工程研究,2022,26(16):2504-2509. [70] QU D, ZHU JP, CHILDS HR, et al. Nanofiber-based transforming growth factor-β3 release induces fibrochondrogenic differentiation of stem cells. Acta Biomater. 2019;93:111-122. [71] LI D, MA X, ZHAO T. Mechanism of TGF-β3 promoting chondrogenesis in human fat stem cells. Biochem Biophys Res Commun. 2020;530(4):725-731. [72] GUHA THAKURTA S, BUDHIRAJA G, SUBRAMANIAN A. Growth factor and ultrasound-assisted bioreactor synergism for human mesenchymal stem cell chondrogenesis. J Tissue Eng. 2015;6:2041731414566529. [73] HUANG L, YI L, ZHANG C, et al. Synergistic effects of FGF-18 and TGF-β3 on the chondrogenesis of human adipose-derived mesenchymal stem cells in the pellet culture. Stem Cells Int. 2018;2018:7139485. [74] 刘登榜,韩小松,黄文良,等.TGF-β3 诱导大鼠骨髓间充质干细胞成软骨细胞分化的实验观察[J].中华医学杂志,2017,97(36):2860-2865 [75] LI J, ZHAO Z, LIU J, et al. MEK/ERK and p38 MAPK regulate chondrogenesis of rat bone marrow mesenchymal stem cells through delicate interaction with TGF-beta1/Smads pathway. Cell Prolif. 2010;43(4):333-343. [76] MCMAHON LA, PRENDERGAST PJ, CAMPBELL VA. A comparison of the involvement of p38, ERK1/2 and PI3K in growth factor-induced chondrogenic differentiation of mesenchymal stem cells. Biochem Biophys Res Commun. 2008;368(4):990-995. [77] DUSFOUR G, MAUMUS M, CAÑADAS P, et al. Mesenchymal stem cells-derived cartilage micropellets: a relevant in vitro model for biomechanical and mechanobiological studies of cartilage growth. Mater Sci Eng C Mater Biol Appl. 2020;112:110808. [78] SHEN H, LIN H, SUN AX, et al. Acceleration of chondrogenic differentiation of human mesenchymal stem cells by sustained growth factor release in 3D graphene oxide incorporated hydrogels. Acta Biomater. 2020;105:44-55. |
| [1] | 农复香, 蒋志雄, 李英豪, 许文聪, 施智兰, 罗 慧, 张晴朗, 钟 爽, 唐梅文. 外泌体调控铁死亡在疾病诊断治疗中的应用与作用[J]. 中国组织工程研究, 2023, 27(在线): 1-10. |
| [2] | 孙可欣, 曾今实, 李佳, 蒋海越, 刘霞. 力学刺激提高生物3D打印软骨构建物基质的形成[J]. 中国组织工程研究, 2023, 27(在线): 1-7. |
| [3] | 潘钟杰, 秦志鸿, 郑铁军, 丁晓飞, 廖世杰. 股骨头坏死发病机制中非编码RNA的靶标性[J]. 中国组织工程研究, 2023, 27(9): 1441-1447. |
| [4] | 蔡志浩, 谢召勇. 股骨颈前倾角测量评估:如何建立统一的方法和标准[J]. 中国组织工程研究, 2023, 27(9): 1448-1454. |
| [5] | 党 祎, 杜成砚, 姚红林, 袁能华, 曹 金, 熊 山, 张顶梅, 王 信. 激素型骨坏死与氧化应激[J]. 中国组织工程研究, 2023, 27(9): 1469-1476. |
| [6] | 聂晨晨, 苏凯奇, 高 静, 凡勇福, 阮晓迪, 袁 洁, 段昭远, 冯晓东. 环状RNA调控脑缺血发病的作用与机制[J]. 中国组织工程研究, 2023, 27(8): 1286-1291. |
| [7] | 高 煜, 韩佳慧, 葛 新. 脊髓缺血再灌注损伤后的免疫炎性微环境[J]. 中国组织工程研究, 2023, 27(8): 1300-1305. |
| [8] | 杨芷姗, 唐正龙. Hippo信号通路中的核心因子YAP/TAZ参与骨形成的作用与机制[J]. 中国组织工程研究, 2023, 27(8): 1264-1271. |
| [9] | 王 继, 张 敏, 杨中亚, 张 龙. 体力活动干预2型糖尿病肌少症的研究现状[J]. 中国组织工程研究, 2023, 27(8): 1272-1277. |
| [10] | 宋荷花, 魏在荣. 糖尿病的周围神经病变:研究与治疗[J]. 中国组织工程研究, 2023, 27(8): 1278-1285. |
| [11] | 徐星星, 文超举, 孟茂花, 王勤英, 陈镜桥, 董 强. 口腔种植中的碳纳米材料[J]. 中国组织工程研究, 2023, 27(7): 1062-1070. |
| [12] | 杨怡天, 王 璐, 姚 蔚, 赵 彬. 生物支架与巨噬细胞在骨再生中的相互影响及应用[J]. 中国组织工程研究, 2023, 27(7): 1071-1079. |
| [13] | 李 诚, 郑国爽, 蒯贤东, 于炜婷. 海藻酸盐支架修复关节软骨[J]. 中国组织工程研究, 2023, 27(7): 1080-1088. |
| [14] | 陈世崧, 刘晓红, 徐志云. 人工生物瓣膜的研究现状及展望[J]. 中国组织工程研究, 2023, 27(7): 1096-1102. |
| [15] | 芦 笛, 张 成, 段荣泉, 刘宗响. 磷酸钙陶瓷骨修复材料的骨诱导性能[J]. 中国组织工程研究, 2023, 27(7): 1103-1109. |
软骨细胞根据其组成结构分为纤维软骨细胞、透明软骨细胞和弹性软骨细胞。位于胫股关节内的半月板属于纤维软骨细胞,其主要合成Ⅰ型胶原和蛋白聚糖[3]。此外,半月板损伤和结构紊乱可引起膝关节疼痛、肿胀和屈曲功能减退等症状[4],且半月板损伤后不能自行愈合,因此针对半月板损伤的治疗成为当下骨科研究热点。关于半月板损伤和退化的治疗,目前常采用的治疗方法是半月板部分或全切、保守治疗和对损伤部位进行缝合,但这些方案治疗效果不佳,且可引起骨关节炎等并发症。半月板游离缘侧无血管、神经和淋巴分布,营养成分主要从关节液渗透获取,故损伤后的自我修复能力有限[5]。因此,对于半月板损伤的治疗急需探索一种新型方案,恢复其天然组织结构和生理功能。
组织工程理论的出现为半月板损伤修复提供新方法[6]。组织工程技术主要包括3个要素:种子细胞、支架和细胞因子[7]。
骨髓间充质干细胞和软骨细胞是半月板组织工程中被研究较多的种子细胞。其中,软骨细胞来源数量有限,获取软骨细胞的同时要对机体产生损伤,且成熟组织细胞易衰老和去分化,多次培养传代后将丢失软骨细胞表型[8]。而与之相比较,骨髓间充质干细胞是一种成体干细胞,其来源充足、可再生、经多次传代后仍具有多向分化能力,基本无免疫排斥,并可规避伦理限制的困扰,因而被作为种子细胞应用于纤维软骨组织工程修复半月板的研究。有报道指出,在半月板组织损伤部位植入骨髓间充质干细胞,其在受损软骨组织中展现出极强的修复能
力[9]。在诸多生长因子中,转化生长因子β(transforming growth factor beta,TGF-β)诱导骨髓间充质干细胞分化为软骨细胞的作用较强[10],可促进合成蛋白聚糖和胶原蛋白等细胞外基质,进而促进半月板受损纤维软骨组织结构重建,修复损伤半月板组织,在一定程度上恢复半月板的生理功能。
目前已有TGF-β诱导骨髓间充质干细胞分化的相关综述,但研究TGF-β诱导骨髓间充质干细胞分化为软骨细胞的综述较少,且这些综述中未指出诱导分化的细胞属于那种软骨细胞系。另外,此前还有研究报道TGF-β1体外诱导人骨髓间充质干细胞定向分化为成纤维细胞的研究,而该综述主要介绍的是TGF-β诱导骨髓间充质干细胞分化为半月板纤维软骨细胞和诱导分化的通路,其中半月板纤维软骨细胞具有合成分泌蛋白聚糖的功能,而成纤维细胞则不具有此作用。该综述还介绍TGF-β1和TGF-β3诱导骨髓间充质干细胞向软骨细胞分化,且指出分化的细胞属于哪种软骨细胞系,并详细介绍TGF-β诱导骨髓间充质干细胞分化为软骨细胞的信号通路和其信号通路的重要组成部分,为纤维软骨组织工程在未来临床中的应用提供了理论基础。 中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
1.1.1 检索人及检索时间 第一作者在2022年3月应用计算机进行检索。
1.1.2 检索文献时限 1990年1月至2022年3月。
1.1.3 检索数据库 PubMed、万方医学网和中国知网数据库。
1.1.4 检索词 英文检索词:“Transforming Growth Factor beta 1,Transforming Growth Factor beta 3,Bone Marrow Mesenchymal Stem Cells,meniscus,fibrochondrocyte”,中文检索词:“TGF-β1、TGF-β3、骨髓间充质干细胞、半月板、纤维软骨细胞”。
1.1.5 检索文献类型 研究原著和综述。
1.1.6 手工检索情况 无。
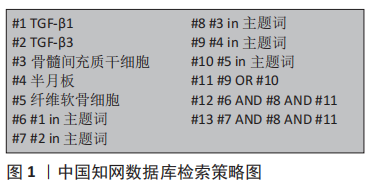
1.1.7 检索策略 以中国知网数据库为例,见图1。
1.2 入组标准
1.2.1 纳入标准 与TGF-β1、TGF-β3、骨髓间充质干细胞、半月板和纤维软骨细胞研究相关的文献。
1.2.2 排除标准 研究内容重复的文献。
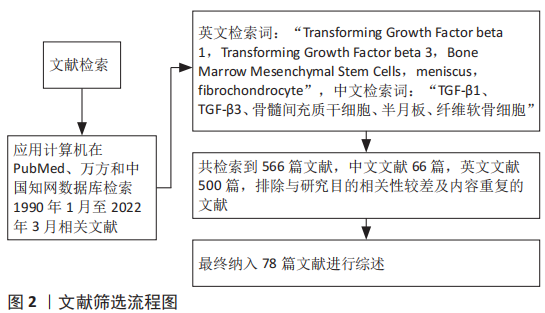
1.3 文献质量评估及数据的提取 共检索到566篇文献,其中中文文献66篇,英文文献500篇,排除与研究目的相关性较差及内容重复的文献,纳入78篇符合标准的文献进行综述,见图2。
TGF-β对间充质干细胞的作用包括:诱导骨髓间充质干细胞向半月板细胞表型分化、促进细胞的增殖生长、促进胶原蛋白和蛋白聚糖的合成分泌。在TGF-β的3种亚型中,最常研究的是TGF-β1和TGF-β3两种亚型。TGF-β1具有诱导骨髓间充质干细胞分化为透明软骨细胞的能力,可促进胶原蛋白和蛋白聚糖表达,抑制Ⅰ型胶原的表达。TGF-β3可诱导骨髓间充质干细胞向着纤维软骨细胞分化[77-78],TGF-β3在诱导分化过程中,可起到促进干细胞迁移聚集、促进纤维软骨细胞外基质Ⅰ型胶原和蛋白聚糖合成分泌作用。纤维软骨细胞主要合成Ⅰ型胶原和蛋白聚糖。
目前,大多数文献对骨髓间充质干细胞诱导分化后的细胞进行鉴定,检测发现有Ⅰ型胶原和蛋白聚糖表达升高,推测诱导分化的细胞是纤维软骨细胞,缺乏从多个方向证实诱导分化的细胞属于纤维软骨细胞,因此还需研究人员不断探索发现。已有研究表明,在骨髓间充质干细胞诱导分化为软骨细胞的过程中,TGF-β1的最佳诱导质量浓度为10 ng/mL,TGF-β3的最佳诱导剂量需要进一步的实验探索研究。TGF-β诱导骨髓间充质干细胞分化为软骨细胞的机制已取得一定的研究进展,但是TGF-β诱导软骨细胞分化尚处于体外和动物实验阶段,与人体内部环境相差较大,因此需要研究者在后续实验中尽可能创造与人体内部环境相似的细胞环境,才能更好地精准调控TGF-β诱导骨髓间充质干细胞分化为半月板纤维软骨细胞,更好地构建组织工程学纤维软骨组织,为未来临床上治疗半月板损伤开发出更完善的方案。
3.2 作者综述区别于他人他篇的特点 目前已有文献对TGF-β1和TGF-β3诱导骨髓间充质干细胞分化为软骨细胞进行了研究,但大部分综述文献并未说明TGF-β1和TGF-β3在诱导骨髓间充质干细胞分化为软骨细胞上的区别,且文献未指出TGF-β诱导骨髓间充质干细胞分化为具体哪一类软骨细胞系。而该综述详细介绍了TGF-β1和TGF-β3诱导骨髓间充质干细胞分化为软骨细胞,并指出TGF-β1有较强诱导骨髓间充质干细胞分化为透明软骨细胞的作用,而TGF-β3则具有较佳诱导骨髓间充质干细胞分化为纤维软骨细胞的功能,并介绍了TGF-β诱导骨髓间充质干细胞分化为软骨细胞的机制和信号通路中的重要物质,这是该综述与其他综述的区别。
3.3 综述的局限性 此综述在检索文献的过程中,筛选研究性文献和综述文献标准不相同,可能存在一些误差,应用参考文献不多,该综述缺乏一定的创新性等问题。文章介绍了TGF-β诱导骨髓间充质干细胞分化为软骨细胞的研究进展,但目前还处于体外和动物模型上,因此需要研究者创造与人体内部相似的环境,并进行大量的研究探索。
3.4 综述的重要意义 文章对近年来发表TGF-β诱导骨髓间充质干细胞分化为软骨细胞做了介绍,并详细介绍了TGF-β1和TGF-β3诱导软骨细胞分化的不同,为半月板纤维软骨组织工程在诱导因子选择上提供了理论依据,并可使后续研究者在较短时间内了解该领域。
3.5 课题专家组对未来的建议 TGF-β诱导骨髓间充质干细胞分化为纤维软骨细胞,其纤维软骨组织工程的应用可为未来临床上半月板损伤的治疗提供一个新方向,但目前关于这方面的研究还处于体外和动物实验阶段,且TGF-β诱导纤维软骨细胞分化的机制较复杂,如何精确地调控TGF-β诱导骨髓间充质干细胞向着纤维软骨细胞分化,以及如何维持分化后的纤维软骨细胞的稳定,这些问题都需要未来的研究者不断探索研究。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程

文题释义:#br# 转化生长因子β:一种刺激骨髓间充质干细胞发育和分化的多肽生长因子,在诱导骨髓间充质干细胞分化为半月板纤维软骨细胞的过程中发挥多种生物学活性,例如参与到细胞迁移聚集、生长增殖和分化等多个过程。#br# 骨髓间充质干细胞:一种来源于骨髓的成体干细胞,具有强大的增殖能力和多向分化潜能,在特定诱导条件下,可分化为成骨细胞、软骨细胞、脂肪细胞、肝细胞和神经细胞等。#br# 中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
软骨细胞根据其组成结构分为纤维软骨细胞、透明软骨细胞和弹性软骨细胞。位于胫股关节内的半月板属于纤维软骨细胞,其主要合成I型胶原和蛋白聚糖。此外,半月板损伤和结构紊乱可引起膝关节疼痛、肿胀和屈曲功能减退等症状,且半月板损伤后不能自行愈合,因此针对半月板损伤的治疗,成为当下骨科研究热点。关于半月板损伤和退化的治疗,目前常采用的治疗是半月板部分或全切、保守治疗和对损伤部位进行缝合,但这些方案治疗效果不佳,且可引起骨关节炎等并发症。半月板游离缘侧无血管、神经和淋巴分布,营养成分主要从关节液渗透获取,故损伤后的自我修复能力有限[5]。因此,对于半月板损伤的治疗急需探索一种新兴治疗方案,恢复其天然组织结构和生理功能。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||