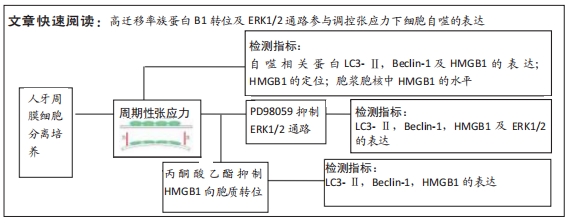
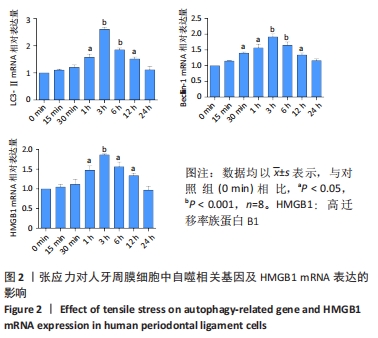
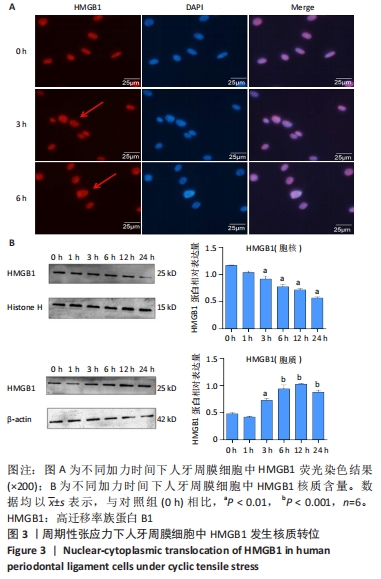
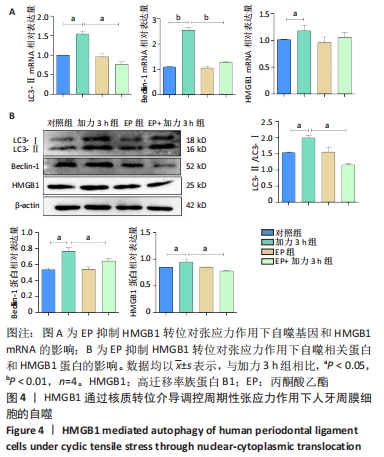
[1] UHLIR R, MAYO V, LIN PH, et al. Biomechanical characterization of the periodontal ligament: Orthodontic tooth movement. Angle Orthod. 2017;87(2):183-192.
[2] LI Y, JACOX LA, LITTLE SH, et al. Orthodontic tooth movement: The biology and clinical implications. Kaohsiung J Med Sci. 2018;34(4):207-214.
[3] 尹圆圆,马华钰,李昕怡,等.小鼠正畸牙移动中牙周组织自噬相关基因表达的初步研究[J].国际口腔医学杂志,2020,47(6):627-634.
[4] LIU Y, AI Y, SUN X, et al. Interleukin-20 Acts as a Promotor of Osteoclastogenesis and Orthodontic Tooth Movement. Stem Cells Int. 2021;2021:5539962.
[5] DUTRA EH, NANDA R, YADAV S. Bone Response of Loaded Periodontal Ligament. Curr Osteoporos Rep. 2016;14(6):280-283.
[6] LIN J, HUANG J, ZHANG Z, et al. Periodontal ligament cells under mechanical force regulate local immune homeostasis by modulating Th17/Treg cell differentiation. Clin Oral Investig. 2022;26(4):3747-3764.
[7] JIN Y, DING L, DING Z, et al. Tensile force-induced PDGF-BB/PDGFRβ signals in periodontal ligament fibroblasts activate JAK2/STAT3 for orthodontic tooth movement. Sci Rep. 2020;10(1):11269.
[8] CASSIDY LD, NARITA M. Autophagy at the intersection of aging, senescence, and cancer. Mol Oncol. 2022. doi: 10.1002/1878-0261. 13269. Online ahead of print.
[9] 陈辛玲,王生兰.细胞自噬过程、通路、调控及其与肺动脉高压的多重相关性[J].中国组织工程研究,2021,25(2):311-316.
[10] DUAN R, XIE H, LIU ZZ. The Role of Autophagy in Osteoarthritis. Front Cell Dev Biol. 2020;8:608388.
[11] ZHANG Y, WANG H, LI F, et al. Inhibitory effects of Dulcitol on rat C6 glioma by regulating autophagy pathway. Nat Prod Res. 2020;34(10): 1437-1441.
[12] ZHANG HL, ZHU YM, ZHOU XY. Coordination of Autophagy and Other Cellular Activities. Adv Exp Med Biol. 2019;1206:697-727.
[13] 蒋玉坤,胡芝爱,关禹哲,等.应力诱导自噬的机械转导过程研究进展[J].四川大学学报(医学版),2021,52(6):929-935.
[14] MEMMERT S, NOGUEIRA AVB, DAMANAKI A, et al. Regulation of the autophagy-marker Sequestosome 1 in periodontal cells and tissues by biomechanical loading. Regulation des Autophagie-Markers Sequestosom 1 durch biomechanische Kräfte in parodontalen Zellen und Geweben. J Orofac Orthop. 2020;81(1):10-21.
[15] MEMMERT S, DAMANAKI A, WEYKOPF B, et al. Autophagy in periodontal ligament fibroblasts under biomechanical loading. Cell Tissue Res. 2019;378(3):499-511.
[16] YANG H, WANG H, ANDERSSON U. Targeting Inflammation Driven by HMGB1. Front Immunol. 2020;11:484.
[17] 周冬梅.高迁移率组蛋白1相关自噬在支气管哮喘中的作用研究[D].沈阳:中国医科大学,2018.
[18] HATAYAMA K, STONESTREET BS. High mobility group box-1 protein as a therapeutic target in perinatal hypoxic-ischemic brain injury. Neural Regen Res. 2021;16(10):2006-2007.
[19] FOGLIO E, PELLEGRINI L, GERMANI A, et al. HMGB1-mediated apoptosis and autophagy in ischemic heart diseases. Vasc Biol. 2019; 1(1):H89-H96.
[20] QU L, CHEN C, CHEN Y, et al. High-Mobility Group Box 1 (HMGB1) and Autophagy in Acute Lung Injury (ALI): A Review. Med Sci Monit. 2019;25:1828-1837.
[21] XU T, JIANG L, WANG Z. The progression of HMGB1-induced autophagy in cancer biology. Onco Targets Ther. 2018;12:365-377.
[22] ZOU Y, XU L, LIN H. Stress overload-induced periodontal remodelling coupled with changes in high mobility group protein B1 during tooth movement: an in-vivo study. Eur J Oral Sci. 2019;127(5):396-407.
[23] WOLF M, LOSSDÖRFER S, ABUDUWALI N, et al. Potential role of high mobility group box protein 1 and intermittent PTH (1-34) in periodontal tissue repair following orthodontic tooth movement in rats. Clin Oral Investig. 2013;17(3):989-997.
[24] NIE F, ZHANG W, CUI Q, et al. Kaempferol promotes proliferation and osteogenic differentiation of periodontal ligament stem cells via Wnt/β-catenin signaling pathway. Life Sci. 2020;258:118143.
[25] XIANG W, JIANG T, HAO X, et al. Primary cilia and autophagy interaction is involved in mechanical stress mediated cartilage development via ERK/mTOR axis. Life Sci. 2019;218:308-313.
[26] XI X, ZHAO Y, LIU H, et al. Nrf2 activation is involved in osteogenic differentiation of periodontal ligament stem cells under cyclic mechanical stretch. Exp Cell Res. 2021;403(2):112598.
[27] TSUGE A, NODA K, NAKAMURA Y. Early tissue reaction in the tension zone of PDL during orthodontic tooth movement. Arch Oral Biol. 2016; 65:17-25.
[28] XI X, LI Z, LIU H, et al. Nrf2 Activation Is Involved in Cyclic Mechanical Stress-Stimulated Osteogenic Differentiation in Periodontal Ligament Stem Cells via PI3K/Akt Signaling and HO1-SOD2 Interaction. Front Cell Dev Biol. 2022;9:816000.
[29] WANG Y, DU C, WAN W, et al. shRNA knockdown of integrin-linked kinase on hPDLCs migration, proliferation, and apoptosis under cyclic tensile stress. Oral Dis. 2020;26(8):1747-1754.
[30] LI S, LI Q, ZHU Y, et al. GDF15 induced by compressive force contributes to osteoclast differentiation in human periodontal ligament cells. Exp Cell Res. 2020;387(1):111745.
[31] WEI W, AN Y, AN Y, et al. Activation of autophagy in periodontal ligament mesenchymal stem cells promotes angiogenesis in periodontitis. J Periodontol. 2018;89(6):718-727.
[32] DU L, LI Y, LIU W. Maresin 1 regulates autophagy and inflammation in human periodontal ligament cells through glycogen synthase kinase-3β/β-catenin pathway under inflammatory conditions. Arch Oral Biol. 2018;87:242-247.
[33] RUSSELL RC, GUAN KL. The multifaceted role of autophagy in cancer. EMBO J. 2022 May 10:e110031. doi: 10.15252/embj.2021110031. Online ahead of print.
[34] DIKIC I, ELAZAR Z. Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cell Biol. 2018;19(6):349-364.
[35] WANG K. Autophagy and apoptosis in liver injury. Cell Cycle. 2015; 14(11):1631-1642.
[36] HOSAKOTE YM, BRASIER AR, CASOLA A, et al. Respiratory Syncytial Virus Infection Triggers Epithelial HMGB1 Release as a Damage-Associated Molecular Pattern Promoting a Monocytic Inflammatory Response. J Virol. 2016;90(21):9618-9631.
[37] YU H, ZHOU W, ZHONG Z, et al. High-mobility group box chromosomal protein-1 deletion alleviates osteoporosis in OVX rat model via suppressing the osteoclastogenesis and inflammation. J Orthop Surg Res. 2022;17(1):232.
[38] ZHU K, ZHU X, LIU S, et al. Glycyrrhizin Attenuates Hypoxic-Ischemic Brain Damage by Inhibiting Ferroptosis and Neuroinflammation in Neonatal Rats via the HMGB1/GPX4 Pathway. Oxid Med Cell Longev. 2022;2022:8438528.
[39] CHEN R, KANG R, TANG D. The mechanism of HMGB1 secretion and release. Exp Mol Med. 2022;54(2):91-102.
[40] LI J, ZHOU W, MAO Q, et al. HMGB1 Promotes Resistance to Doxorubicin in Human Hepatocellular Carcinoma Cells by Inducing Autophagy via the AMPK/mTOR Signaling Pathway. Front Oncol. 2021; 11:739145.
[41] MENG Q, PU L, QI M, et al. Laminar shear stress inhibits inflammation by activating autophagy in human aortic endothelial cells through HMGB1 nuclear translocation. Commun Biol. 2022;5(1):425.
[42] ANDERSSON U, YANG H, HARRIS H. High-mobility group box 1 protein (HMGB1) operates as an alarmin outside as well as inside cells. Semin Immunol. 2018;38:40-48.
[43] Zhao M, Zhang Y, Jiang Y, et al. YAP promotes autophagy and progression of gliomas via upregulating HMGB1. J Exp Clin Cancer Res. 2021;40(1):99.
[44] LEI T, HUANG J, XIE F, et al. HMGB1-mediated autophagy promotes gefitinib resistance in human non-small cell lung cancer. Acta Biochim Biophys Sin (Shanghai). 2022;54(4). doi: 10.3724/abbs.2022023. Online ahead of print.
[45] RAUCCI A, DI MAGGIO S, SCAVELLO F, et al. The Janus face of HMGB1 in heart disease: a necessary update. Cell Mol Life Sci. 2019;76(2):211-229.
[46] TANG D, KANG R, ZEH HJ 3RD, et al. High-mobility group box 1, oxidative stress, and disease. Antioxid Redox Signal. 2011;14(7):1315-1335.
[47] YIN H, YANG X, GU W, et al. HMGB1-mediated autophagy attenuates gemcitabine-induced apoptosis in bladder cancer cells involving JNK and ERK activation. Oncotarget. 2017;8(42):71642-71656.
[48] ZHU L, HUANG G, SHENG J, et al. High-mobility group box 1 induces neuron autophagy in a rat spinal root avulsion model. Neuroscience. 2016;315:286-295.
[49] MA C, CHEN H, ZHANG S, et al. Exosomal and extracellular HMGB1 have opposite effects on SASH1 expression in rat astrocytes and glioma C6 cells. Biochem Biophys Res Commun. 2019;518(2):325-330.
[50] KIM YH, KWAK MS, LEE B, et al. Secretory autophagy machinery and vesicular trafficking are involved in HMGB1 secretion. Autophagy. 2021;17(9):2345-2362.
[51] AOYAGI H, YAMASHIRO K, HIRATA-YOSHIHARA C, et al. HMGB1-induced inflammatory response promotes bone healing in murine tooth extraction socket. J Cell Biochem. 2018;119(7):5481-5490.
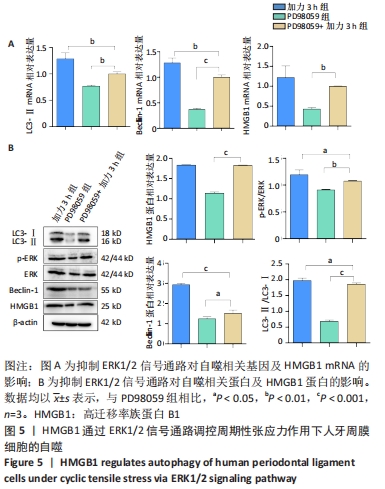
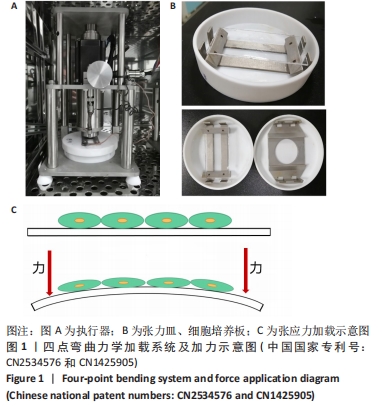
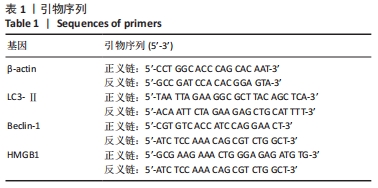
|