Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (8): 1295-1300.doi: 10.12307/2024.207
Previous Articles Next Articles
Research progress in gout-induced bone destruction mechanism
Lin Zeyu1, Xu Lin2
- 1The Second Clinical College of Binzhou Medical University, Yantai 264000, Shandong Province, China; 2Yantai Affiliated Hospital of Binzhou Medical University, Yantai 264000, Shandong Province, China
-
Received:2023-02-10Accepted:2023-03-18Online:2024-03-18Published:2023-07-19 -
Contact:Xu Lin, MD, Chief physician, Yantai Affiliated Hospital of Binzhou Medical University, Yantai 264000, Shandong Province, China -
About author:Lin Zeyu, Master candidate, The Second Clinical College of Binzhou Medical University, Yantai 264000, Shandong Province, China -
Supported by:the Natural Science Foundation of Shandong Province, No. ZR2018LH003 (to XL)
CLC Number:
Cite this article
Lin Zeyu, Xu Lin. Research progress in gout-induced bone destruction mechanism[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1295-1300.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks

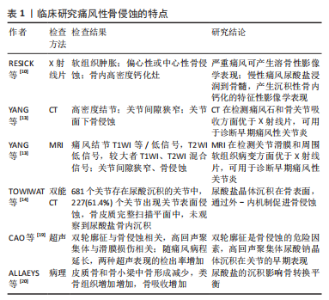
临床研究(影像、组织病理)通过特征性表现阐释了痛风性骨侵蚀的特点,各类前瞻性、回顾性对照研究揭示了尿酸钠晶体与骨侵蚀的强相关性,为临床痛风的诊断和治疗提供指导。基础研究发现,痛风性骨破坏是尿酸钠晶体在促吸收因子和RANKL协助下诱导破骨样细胞分化的结果,骨保护素(OPG)、硬化蛋白参与修复性骨重塑的过程。在痛风性破坏的发病机制中,尿酸钠晶体对骨细胞、软骨细胞、成骨细胞的直接刺激作用占据主要地位,各类炎性因子的爆发性聚集、释放RANKL/RANK通路,引起单核/巨噬细胞朝向多核破骨样细胞分化,引起骨吸收效应。在此通路中,T细胞是RANKL的主要载体,白细胞介素1等炎症因子、RANKL/RANK、尿酸钠晶体相互通过协同刺激作用促进骨破坏和重塑。此外,中性粒细胞和其吞噬体中性粒细胞外诱捕网(neutrophil extracellular trap networks,NETS)也参与破骨活动。 2.1 从组织学和影像学阐述痛风石骨质破坏 痛风患者放射学检查主要表现为尿酸盐沉积样改变和多发性骨侵蚀。RESNICK等[10]早期的回顾性研究发现,慢性痛风患者的X射线片提示存在高密度钙化灶,经病理观察后发现这种钙化灶是尿酸钠晶体的骨内沉积。早期痛风X射线平片检测骨侵蚀敏感性低,通常表现正常骨质影像学特点,慢性痛风患者(通常7-10年)X射线平片提示边缘穿孔、关节和关节旁侵蚀,晚期痛风可发现关节间隙增宽,关节周围巨大溶骨性改变[11]。 CARTER等[12]利用核磁共振(magnetic resonance imaging,MRI)和超声访视了27例痛风患者的指数关节(痛风急性发作时疼痛最严重的关节),MRI发现15例(56%)患者存在指数关节骨侵蚀(P < 0.000 1),超声仅发现1例(4%)患者存在骨侵蚀(P=NS),这些痛风患者的指数关节在X射线平片上表现正常,表明大部分X射线平片表现正常的痛风患者存在隐秘性关节破坏,MRI检测X射线阴性的亚临床骨侵蚀有较高的敏感性。计算机断层扫描(computed tomography,CT)和MRI可直接显示痛风结节沉积和骨质侵蚀,YANG等[13]回顾性分析了14例肩关节、肘关节痛风患者的MRI和CT影像学特点,其中7例患者接受CT扫描,发现主要表现为病灶区高密度结节、关节间隙狭窄、关节面下骨侵蚀;7例患者接受MRI检查,主要表现为病灶结节呈T1等信号/低信号,T2高信号,较大的痛风结节表现T1、T2混合信号、关节间隙狭窄、关节面骨侵蚀,MRI和CT在显示关节间隙狭窄、关节内骨侵蚀、周围软组织肿胀无差异(P < 0.05),MRI对显示关节积液有更高的敏感性(P < 0.05)。TOWIWAT等[14]利用双能CT对144例痛风患者的1 433块跖骨头评分,发现681个关节存在尿酸晶体沉积,其中370个(54.3%)存在尿酸盐与骨骼接触的情况,这些接触的情况中,有227个关节面存在侵蚀(61.4%),未发现真正存在的骨内部沉积(P < 0.000 1),这项研究表明尿酸盐沉积在关节内、骨表面和骨内部,支持尿酸盐通过“外-内”机制促进骨侵蚀的概念。MCQUEEN等[15]报道了798个痛风性手关节和腕关节的CT和X射线扫描情况,98个关节X射线扫描发现大型骨质侵蚀,其中96个关节CT发现存在痛风结节(98%);CT显示56个关节存在较大骨质侵蚀(直径> 7.5 mm),其中所有关节均发现痛风结节(100%),表明痛风结节与骨侵蚀有强相关性(研究中未给出r值);MCQUEEN等还发现CT显示的骨质侵蚀的直径与骨内痛风有强相关性(r=0.93)。 超声是一种无创、无电离辐射、方便且廉价的方法,现已被用于鉴定尿酸盐沉积和诊断痛风,发现透明软骨表面的不规则增强,即“双轮廓征”,是痛风晶体沉积并侵犯关节的证据 [16-18]。CAO等[19]利用超声检测了202例痛风患者的1 082个关节,结果表明超声下双轮廓征和痛风石是骨侵蚀的危险因素;检测了43例高尿酸血症患者的256个关节,结果表明超声下高回声聚集体(HAG)是尿酸钠晶体沉积的早期表现。 在组织病理学研究中,痛风结节的主要特征是侵蚀性尿酸钠晶体的散在性沉积(包括骨骼结构在内的各种组织内,呈白色粉末或白色结节状);经过苏木精-伊红染色后,偏振光镜下观察到骨髓腔内和骨皮质旁存在透明色、无定形样晶体;经四色染色后,偏振光镜下观察到灰蓝色尿酸盐晶体被炎性组织包裹,周围存在类骨样增生,不规则地被板层骨包围;成骨细胞广泛回缩,失去栅栏样规则排列的形式,骨吸收区发现破骨细胞(图2)[20]。迪夫染色后,偏振光镜观察到大量针状、明亮黄色尿酸晶在细胞内外广泛分布,呈双折射效应[21]。以上病理学研究表明,痛风病变中尿酸盐结晶以及周围组织,痛风晶体破坏骨质可深达骨髓,无规律性沉积、广泛分布,周围发现收缩的成骨细胞、活化的破骨细胞。"

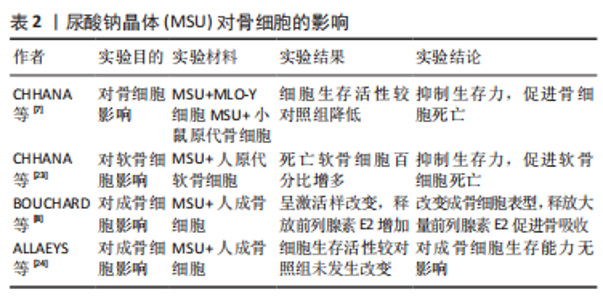
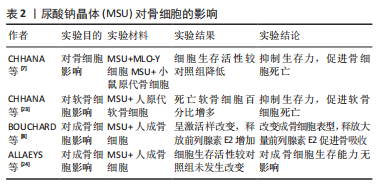
2.2 尿酸钠晶体对骨破坏的影响 痛风患者的骨侵蚀与痛风数量、痛风持续时间密切相关,与痛风石大小无关。一项涉及980例痛风患者的多变量逻辑回归分析表明,痛风结石是骨侵袭最强的相关因素(OR=4.218,95%置信区间3.092-5.731),骨侵蚀风险随痛风石数量增加而提高(P < 0.001)[14]。CHHANA等[22]研究发现,痛风石数量与CT侵蚀评分呈正相关;对骨侵蚀影响因素路径分析表明,痛风结石数量不但可以直接影响CT骨侵蚀评分(P < 0.000 1),还可以通过骨保护素(P=0.035)、RANKL(P=0.0021)和硬化蛋白(P=0.044)等介质影响CT骨侵蚀评分,上述关联性研究表明,痛风石数量对骨侵蚀直接相关,也可以通过RANKL间接促进骨侵蚀;骨保护素、硬化蛋白等调节因子促进代偿修复机制。 2.2.1 尿酸钠晶体对骨细胞的影响 CHHANA等[7]利用小鼠原代骨细胞和MLO-Y4细胞(替代人体骨细胞)行体外实验,将尿酸钠晶体与上述细胞混合培养,发现高浓度(0.3-0.5 mL/L)的尿酸钠结晶随时间依赖性降低MLO-Y4细胞的活性,这种抑制作用在48 h达到顶峰;此外,通过RCR技术测定相关基因的表达情况,表明尿酸钠晶体不影响骨细胞骨相关基因(骨保护素)和炎症基因(肿瘤坏死因子α、白细胞介素6、白细胞介素11)的表达。表明尿酸钠晶体直接抑制骨细胞活性,诱导骨细胞死亡,并且无法从基因层面影响骨细胞功能。 CHHANA等[23]利用人原代软骨细胞与尿酸钠晶体混合培养,发现尿酸钠晶体以剂量依赖性、时间依赖性降低软骨细胞活力(P < 0.001),诱导软骨细胞死亡;在显微镜下观察到痛风周围软骨丧失正常结构,表面不连续且出现空隙,仅发现少量生存的软骨细胞,这为尿酸钠晶诱导下软骨细胞的死亡作用提供了强有力的证据;此外,尿酸钠晶体沉积能够促进软骨降解酶基因表达,抑制基质蛋白基因表达,导致软骨细胞合成基质蛋白和胶原蛋白减少,造成软骨修复能力下降。尿酸钠晶体通过抑制软骨细胞活力和诱导代谢功能紊乱导致慢性痛风患者软骨损伤,但溶解的尿酸盐不能损伤软骨细胞。这种现象解释了痛风患者更容易产生四肢小关节功能紊乱和畸形的原因:四肢温度较体核温度低,尿酸结晶物理溶解度随温度下降而降低,更容易析出沉积在关节软骨,造成四肢小关节破坏。 ALLAEYS等[24]将成骨细胞与尿酸钠晶体混合培养,发现尿酸钠晶体对成骨细胞生存能力无影响。BOUNCHARD等[8]通过电镜观察到尿酸钠晶体黏附在激活状态的人成骨细胞周围,并且被部分吞噬;动力学实验发现,尿酸钠晶体以剂量依赖性诱导成骨细胞表达环氧合酶2(COX-2),刺激成骨细胞释放前列腺素E2,最高较照组增高35倍(P=0.006);白细胞介素1对增产效应产生显著的协同作用,最高较对照组增产141倍(P=0.04)。前列腺素E2是由环氧合酶催化花生四烯酸产生的重要脂质介质,通过与4种前列腺素视紫红质样G蛋白偶联受体(EP1-4)结合调节血压、炎症、生育功能和骨稳态[25-30]。前列腺素受体2(EP2)和前列腺素受体4(EP4)在破骨细胞中高度表达,前列腺素E2通过前列腺素受体2和前列腺素受体4介导巨噬细胞源性破骨细胞的迁移和分化。JIANG等[31]建立了敲除前列腺素受体2基因和前列腺素受体4基因的骨关节炎小鼠模型,发现小鼠破骨细胞的迁移和分化显著降低。BOUNCHARD等[8]的体外实验发现,与对照组相比,尿酸钠晶体组中成骨细胞释放骨钙素量下降42%(P=0.03),碱性磷酸酶释放量下降38%(P=0.02),上述两种物质对维持骨合成代谢有重要作用。综上所述,尿酸钠晶体通过改变成骨细胞表型,促进成骨细胞释放超量前列腺素E2、抑制成骨细胞释放骨钙素和碱性磷酸酶,促进成骨细胞向抑制骨形成和促进骨吸收方向发展。见表2。"
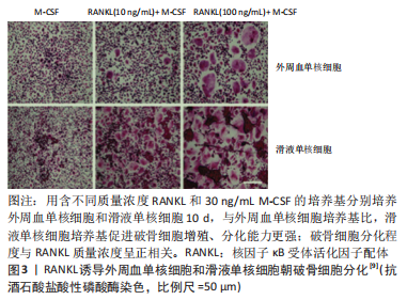
2.2.2 尿酸钠晶体对骨吸收因子的影响 LEE 等[9]用尿酸晶体刺激健康受试者的外周血单核细胞,利用PCR技术测定吸收因子表达情况,发现尿酸钠晶体呈时间依赖性、剂量依赖性增强白细胞介素1α、白细胞介素1β、白细胞介素6、肿瘤坏死因子α和RANKL等骨吸收因子表达;随后通过PCR技术发现,白细胞介素1α、白细胞介素1β全部来自单核细胞,白细胞介素6由单核细胞和T细胞共同产生。上述实验表明尿酸钠晶体具有直接诱导促吸收因子释放的能力,揭示了各类骨吸收因子的来源。CHOE等[32]通过动物实验发现,尿酸钠晶体和RANKL均可促进RAW264.7细胞表达RANK/RANKL,并且尿酸钠晶体和RANKL与RAW264.7细胞共同培养时,促进RANKL/RANK表达作用显著增强,表明尿酸钠晶体和RANKL协同增强单核细胞表达RANKL/RANK这类重要的促吸收因子。而且RANKL促进自身和其受体(RANK)表达的作用,是一种正反馈机制。CHHANA等[7]通过PCR技术发现,尿酸钠晶体刺激的小鼠单核/巨噬细胞细胞(RAW264.7)培养基显著增强MLO-Y4细胞表达骨相关因子(包括E11、RANKL、连接蛋白43)和炎性递质(包括白细胞介素1、白细胞介素6β、白细胞介素11、肿瘤坏死因子α、环氧合酶22),表明尿酸钠晶体通过与巨噬细胞相互作用,间接促进骨细胞分泌骨吸收因子。 综上所述,尿酸钠晶体既可以直接促进单核细胞、T细胞分泌骨吸收因子,又可以通过RANKL等相关因子激活巨噬细胞,通过巨噬细胞间接促进骨细胞分泌骨吸收因子。 2.3 骨吸收因子对骨破坏的影响 在临床上,RANKL是一种经典骨破坏标志物,田晓玲等[33]测定了63例痛风性关节炎患者的血清骨吸收因子水平,发现较对照组相比,RANKL、白细胞介素6、肿瘤坏死因子α等骨吸收因子均显著升高(P < 0.05)。ZOU等[34]的回顾性研究发现,合并存在痛风石和骨侵蚀的痛风患者体内RANKL水平最高,表明痛风患者体内RANKL水平与骨破坏严重程度密切相关。一项涉及100例痛风患者的前瞻性随机对照试验发现,体内循环RANKL(r=0.44 P=0.001)与侵蚀评分呈正相关;多元回归分析表明,RANKL是痛风性骨侵蚀独立相关因素[35]。以上研究均表明,RANKL与痛风性骨破坏存在显著相关。 HARRE等[36]的研究发现,RANKL具有诱导单核/巨噬细胞朝破骨细胞分化的潜能。LEE等[9]将不同质量浓度的RANKL与痛风性关节炎患者的外周血单核细胞、滑液单核细胞分别混合培养,发现两种现象:①相同培养环境下,滑液单核细胞培养基中白细胞介素1、白细胞介素6、肿瘤坏死因子α等促吸收因子的表达远高于外周血单核细胞,滑液单核细胞培养基比外周血单核细胞培养基产生更多数量的TRAP+多核巨噬细胞,表明促吸收因子促进破骨样细胞分化、成熟,产生破骨效应;②缺乏RANKL的培养基中破骨样细胞分化微弱,TRAP+多核巨噬细胞的分化数量随RNAKL质量浓度升高而增加,表明RANKL具有诱导破骨细胞的分化、成熟的能力,见图3。"

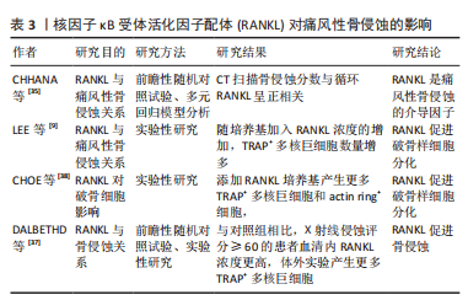
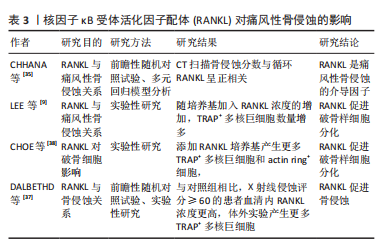
DALBETHD等[37]研究发现,与对照组相比,X射线侵蚀得分≥60分的痛风患者,血清RANKL浓度和TRAP+多核巨噬细胞分化数量均显著增高(P < 0.05),表明血清RANKL浓度可以反映痛风性骨侵蚀严重程度;此外,尿酸钠晶体通过抑制基质细胞骨保护素基因和蛋白表达,改变RANKL-骨保护素轴平衡,RANKL-骨保护素平衡破坏可能促进破骨样细胞产生;RANKL和M-CSF共同培养时,痛风患者的外周血单核细胞具有产生破骨样细胞的能力。CHOE等[38]体外实验发现,与对照组相比,利用RANKL单独诱导、RANKL与尿酸结晶共同诱导RAW264.7细胞,产生更多TRAP+多核巨细胞和acting ring+细胞(RANKL单独诱导P < 0.05,RANKL与尿酸晶体共同诱导P < 0.01),表明RANKL具有促进单核/巨噬细胞分化为破骨样细胞的能力;凹坑形成测定实验发现,TRAP+多核巨细胞和破骨细胞具有相似的形态和功能学特征,产生骨吸收效应。此外,化学分子传递信号参与RANKL诱导破骨细胞分化,CHOE等[38]研究发现,在尿酸钠晶体刺激下,RANKL与RANK结合后,激活TRAF-6和蛋白激酶JNK,诱导单核/巨噬细胞朝TRAP+多核巨噬细胞方向分化;因此,RANKL/RANK通路中,TRAF-6和JNK是NRAKL/RANK通路中产生破骨效应的重要化学分子。RANKL对痛风性骨侵蚀的影响,见表3。"
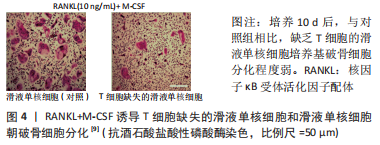
白细胞介素1是一种典型的炎症因子,能强烈促进骨和软骨破坏[39],CHOE等[38]研究发现,敲除白细胞介素1β基因后,TRAF-6、c-jun、NFATc1等参与破骨细胞活化的重要化学分子表达明显降低;TRAP染色实验表明破骨样细胞分化数量也明显降低。白细胞介素1的表达在RANKL/RANK通路中受到尿酸钠晶体和RANKL调节,并且参与RANKL/RANK通路化学信号传递。此外,尿酸钠晶体与脂多糖可以协同促进白细胞介素1β释放[40],激活NLRP3(NOD样受体家族,含有3个pyrin结构域)炎症小体,产生活性白细胞介素1β和白细胞介素18[41],白细胞介素1受体(IL-1R)信号对尿酸钠诱导的痛风性关节炎至关重要[42]。综上所述,尿酸钠晶体和RANKL可以募集并激活白细胞介素1,白细胞介素1通过激活NLRP3炎症小体放大炎症反应,参与RANKL/RANK分子通路介导的破骨效应。白细胞介素1抑制剂是临床痛风治疗的重要靶点,阿纳白滞素、白细胞介素1受体重组抗体、抗白细胞介素1β单克隆抗体可以通过阻断白细胞介素1信号传递,减轻畸形关节炎发作[43-45]。一项涉及64例男性痛风患者和50名男性健康人群的前瞻性随机对照研究发现,痛风患者的血清白细胞介素1β、白细胞介素6、可溶性白细胞介素6(sIL-6)、骨保护素、RANKL存在显著差异,多变量调整回归性分析表明,炎性细胞因子可溶性白细胞介素6和骨保护素与痛风患者放射损伤指数呈负相关[46]。 有学者提出痛风性关节炎骨破坏模型:痛风晶体通过浸润单核/巨噬细胞诱导产生骨吸收因子(如白细胞介素1、白细胞介素6、T肿瘤坏死因子α),并通过激活T细胞诱导RANKL表达,一系列联级炎症反应诱导多核巨细胞分化破骨细胞,产生骨吸收效应[47]。 Dickkopf-1(DKK-1)是一种经典β-catenin/Wnt信号通路的分泌蛋白类抑制剂,抑制间充质干细胞向成骨细胞分化,在调节骨形成、骨吸收和骨转移中起重要作用[48-49]。赵卫等[50]检测了150例痛风性关节炎患者血清DKK-1和TRAP5b水平,发现较对照组相比,痛风性关节炎组患者血清DKK-1水平显著增高(P < 0.01);表明DKK-1作为抑制因子参与了痛风性骨破坏;痛风性关节炎组患者血清DKK-1水平与TRAP5b水平呈正相关(P < 0.000 1)。TRAP5b是反映破骨细胞活性的重要指标,表明血清DKK-1水平可以反映破骨细胞活性,是临床监测痛风活动情况重要指标。GAVRIATOPOULOU等[51]通过构建小鼠骨髓瘤模型的实验发现,DK利用中和性抗DKK-1抗体增加成骨细胞数量,减少破骨细胞数量,增加骨体积,为临床治疗痛风骨破坏提供了新靶点。 2.4 T细胞对骨破坏的影响 为研究T细胞促进RANK/RANKL通路对骨破坏的影响,LEE等[9]体外建立实验组T细胞缺失的滑液单核细胞培养基、对照组含T细胞滑液单核细胞培养基,加入RANKL和M-CSF混合培养,利用TRAP染色计数TRAP+多核巨噬细胞,发现与对照组相比,T细胞缺失的滑液单核细胞培养基中TRAP+多核巨噬细胞细胞数量减少约60%(P=0.028),表明T细胞在破骨细胞的分化中起重要作用(图4)。"

KOTAKE等[47]体外实验发现表达RANKL的激活态T细胞诱导自体单核细胞分化成破骨细胞,而且这种T细胞过度产生RANKL会增加关节滑液游离RANKL(sRANKL)水平,表明激活态T细胞是表达RANKL的主要载体,在破骨细胞生成中起到桥梁作用。CHO等[52]研究发现,痛风晶体能增强MALT细胞中CD69的表达,这被认为是MALT细胞激活的标志,表明痛风晶体能促进MALT细胞激活;此外,还发现血清激活的MAIT细胞(黏膜相关稳定T细胞,T细胞的一种亚型)存在向关节滑液中迁移现象;随后的实验发现,激活态MALT细胞培养基中炎症因子(肿瘤坏死因子α、白细胞介素17、白细胞介素6)蛋白表达、TAAP+多核巨噬细胞数量、吸收坑数量明显高于对照组,表明尿酸钠晶体可以激活T细胞,激活态T细胞表达炎性因子(肿瘤坏死因子α、白细胞介素17、白细胞介素6),促进破骨样细胞分化,产生骨破坏效应;在此通路中存在2个关键环节:T细胞的激活;激活态T细胞向痛风组织、关节滑液的迁移效应。 2.5 中性粒细胞对骨破坏的影响 ALLAEYS等[20]发现在显微镜下中性粒细胞可以将成骨细胞排列规则的长方体形态改变为具有多边形、纺锤形特点的星状形态,这种改变依赖中性粒细胞与成骨细胞直接接触;动力学实验表明,这种形态学影响与中性粒细胞浓度和培养时间成正相关,而且在接触短时间内(48 h),分离两种细胞后,这种效应可以被逆转。随后的实验发现,中性粒细胞的存在促进成骨细胞收缩,这种收缩不会改变成骨细胞基质矿化的数量,可能与骨吸收密切相关;经特殊染色的病理切片发现,中性粒细胞存在痛风骨破坏病变附近,周围成骨细胞丧失栅栏样排列形式而广泛收缩,骨破坏区存在破骨细胞活动;痛风晶体周围增生类骨组织上也发现成骨细胞。有动物实验表明,中性粒细胞能激活破骨细胞、抑制成骨细胞[53]。中性粒细胞改变成骨细胞形态,抑制成骨细胞功能,而收缩的成骨细胞导致内部基质更容易受破骨细胞影响,而产生骨质缺损;骨基质产生不规则钙化,产生类骨组织,主要是因为成骨细胞的在类骨组织中产生成骨作用,中性粒细胞在骨破坏和骨重塑中起重要作用。 NETs是一种中性粒细胞杀灭病原菌的防御机制,这种白细胞引起的自身免疫反应可以抑制体内炎症急性发作。NETs由多形核中性粒细胞颗粒蛋白和细胞外DNA网络构成,NE是NETs的主要活性集群,可以水解各种组织蛋白,痛风引发的NETs可导致局部软骨损伤、周围组织损伤和骨重塑[54-56]。JIA等[57]体外实验研究了NETs导致骨破坏的详细机制,利用NETs上清液处理人成骨细胞,发现成骨细胞活性减弱,碱性磷酸酶和骨保护素相关mRNA表达升高,RANKL相关mRNA表达降低;为了研究NETs对破骨细胞的影响,JIA等将NETs与破骨细胞共同培养,发现破骨细胞活性未发生改变,将NETs与成骨细胞混合培养获得上清液,利用上清液处理破骨细胞,发现破骨细胞分化的标志物(TRAP、RANK、Ctsk)显著增加。以上实验表明NETs不仅可以直接抑制成骨细胞活力,还可以通过RANKL/RANK、骨保护素失衡间接刺激破骨细胞分化。 2.6 基因突变与骨破坏的相关性 D HAYSHI 等[58]报告了一例肥胖合并全身多发痛风石的27岁男性患者,X射线平片和MRI检查发现多处骨破坏病灶,并且发现该患者存在AGCB2基因和SLC16A9/MCT9基因变异。MATSUO等[59]通过全基因组关联研究发现,ABCG2/CBRP是痛风易感基因;报告还指出,78.6%的痛风患者存在ABCG2基因突变,这种基因功能失调会显著增加痛风的发病风险(OR=22.2)。NAKAYAMA等[60]调查了545例痛风患者和1 115名健康受试者MCT9基因突变与痛风之间的关系,发现MCT9基因突变与肾过载性痛风相关,与痛风易感性相关性,SLC16A9/MCT9基因变异能轻度增加痛风发病风险。目前基因方向的研究主要集中在基因突变对痛风易感性影响,暂无文献指出与骨破坏的关系,该研究方向存在空缺。"

| [1] SINGH JA, REDDY SG, KUNDUKULAM J. Risk factors for gout and prevention: a systematic review of the literature. Curr Opin Rheumatol. 2011;23(2):192-202. [2] 高尿酸血症相关疾病诊疗多学科共识专家组. 中国高尿酸血症相关疾病诊疗多学科专家共识[J]. 中华内科杂志,2017,56(3):235-248. [3] DANVE A, SEHRA ST, NEOGI T. Role of diet in hyperuricemia and gout. Best Pract Res Clin Rheumatol. 2021;35(4):101723. [4] FU T, CAO H, YIN R, et al. Associated factors with functional disability and health-related quality of life in Chinese patients with gout: a case-control study. BMC Musculoskelet Disord. 2017;18(1):429. [5] 朱坤智,卢涛,罗张风,等.四肢痛风石外科治疗时机和适应证[J].中国组织工程研究,2021,25(30):4883-4890. [6] LI Q, LI X, WANG J, et al. Diagnosis and treatment for hyperuricemia and gout: a systematic review of clinical practice guidelines and consensus statements. BMJ Open. 2019;9(8):e026677. [7] CHHANA A, POOL B, CALLON KE, et al. Monosodium urate crystals reduce osteocyte viability and indirectly promote a shift in osteocyte function towards a proinflammatory and proresorptive state. Arthritis Res Ther. 2018;20(1):208. [8] BOUCHARD L, DE MÉDICIS R, LUSSIER A, et al. Inflammatory microcrystals alter the functional phenotype of human osteoblast-like cells in vitro: synergism with IL-1 to overexpress cyclooxygenase-2. J Immunol. 2002;168(10):5310-5317. [9] LEE SJ, NAM KI, JIN HM, et al. Bone destruction by receptor activator of nuclear factor κB ligand-expressing T cells in chronic gouty arthritis. Arthritis Res Ther. 2011;13(5):R164. [10] RESNICK D, BRODERICK TW. Intraosseous calcifications in tophaceous gout. AJR Am J Roentgenol. 1981;137(6):1157-1161. [11] BARTHELEMY CR, NAKAYAMA DA, CARRERA GF, et al. Gouty arthritis: a prospective radiographic evaluation of sixty patients. Skeletal Radiol. 1984;11(1):1-8. [12] CARTER JD, KEDAR RP, ANDERSON SR, et al. An analysis of MRI and ultrasound imaging in patients with gout who have normal plain radiographs. Rheumatology (Oxford). 2009;48(11):1442-1446. [13] YANG Y, GUO Y, YU S, et al. Computed tomography and magnetic resonance imaging findings in gouty arthritis involving large joints of the upper extremities. BMC Med Imaging. 2022;22(1):167. [14] TOWIWAT P, DOYLE AJ, GAMBLE GD, et al. Urate crystal deposition and bone erosion in gout: ‘inside-out’ or ‘outside-in’? A dual-energy computed tomography study. Arthritis Res Ther. 2016;18(1):208. [15] MCQUEEN FM, DOYLE A, DALBETH N. Imaging in gout--what can we learn from MRI, CT, DECT and US?. Arthritis Res Ther. 2011;13(6):246. [16] SLOT O, TERSLEV L. Ultrasonographic signs of gout in symmetric polyarthritis. Arthritis Rheum. 2010;62(11):3487. [17] NAREDO E, USON J, JIMÉNEZ-PALOP M, et al. Ultrasound-detected musculoskeletal urate crystal deposition: which joints and what findings should be assessed for diagnosing gout? Ann Rheum Dis. 2014;73(8):1522-1528. [18] NEOGI T, JANSEN TL, DALBETH N, et al. 2015 Gout classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2015;74(10):1789-1798. [19] CAO L, ZHAO T, XIE C, et al. Performance of Ultrasound in the Clinical Evaluation of Gout and Hyperuricemia. J Immunol Res. 2021;2021:5550626. [20] ALLAEYS I, RUSU D, PICARD S, et al. Osteoblast retraction induced by adherent neutrophils promotes osteoclast bone resorption: implication for altered bone remodeling in chronic gout. Lab Invest. 2011;91(6):905-920. [21] KOTAKE S, UDAGAWA N, HAKODA M, et al. Activated human T cells directly induce osteoclastogenesis from human monocytes: possible role of T cells in bone destruction in rheumatoid arthritis patients. Arthritis Rheum. 2001;44(5):1003-1012. [22] CHHANA A, AATI O, GAMBLE GD, et al. Path Analysis Identifies Receptor Activator of Nuclear Factor-κB Ligand, Osteoprotegerin, and Sclerostin as Potential Mediators of the Tophus-bone Erosion Relationship in Gout. J Rheumatol. 2016;43(2):445-449. [23] CHHANA A, CALLON KE, POOL B, et al. The effects of monosodium urate monohydrate crystals on chondrocyte viability and function: implications for development of cartilage damage in gout. J Rheumatol. 2013;40(12):2067-2074. [24] ALLAEYS I, RUSU D, PICARD S, et al. Osteoblast retraction induced by adherent neutrophils promotes osteoclast bone resorption: implication for altered bone remodeling in chronic gout. Lab Invest. 2011;91(6):905-920. [25] ZHANG Y, DAAKA Y. PGE2 promotes angiogenesis through EP4 and PKA Cγ pathway. Blood. 2011;118(19):5355-5364. [26] LU W, YU W, HE J, et al. Reprogramming immunosuppressive myeloid cells facilitates immunotherapy for colorectal cancer. EMBO Mol Med. 2021;13(1):e12798. [27] NI S, LING Z, WANG X, et al. Sensory innervation in porous endplates by Netrin-1 from osteoclasts mediates PGE2-induced spinal hypersensitivity in mice. Nat Commun. 2019;10(1):5643. [28] NAKANISHI M, ROSENBERG DW. Multifaceted roles of PGE2 in inflammation and cancer. Semin Immunopathol. 2013;35(2):123-137. [29] JIN J, TANG Q, LI Z, et al. Prostaglandin E2 regulates renal function in C57/BL6 mouse with 5/6 nephrectomy. Life Sci. 2017;174:68-76. [30] TU M, YANG M, YU N, et al. Inhibition of cyclooxygenase-2 activity in subchondral bone modifies a subtype of osteoarthritis. Bone Res. 2019;7:29. [31] JIANG W, JIN Y, ZHANG S, et al. PGE2 activates EP4 in subchondral bone osteoclasts to regulate osteoarthritis. Bone Res. 2022;10(1):27. [32] CHOE JY, PARK KY, KIM SK. Monosodium Urate in the Presence of RANKL Promotes Osteoclast Formation through Activation of c-Jun N-Terminal Kinase. Mediators Inflamm. 2015;2015:597512. [33] 田晓玲,陈莉,刘满华,等.痛风性关节炎患者NETs表达水平与骨破坏、炎症因子的相关性分析[J]. 广州医科大学学报,2021,49(4):67-70. [34] ZOU Y, FEI Y, GAO H, et al. Association between musculoskeletal ultrasonography and bone remodelling markers and its role in disease monitoring of gout and hyperuricaemia. Clin Exp Rheumatol. 2020;38(5):896-902. [35] CHHANA A, AATI O, GAMBLE GD, et al. Path Analysis Identifies Receptor Activator of Nuclear Factor-κB Ligand, Osteoprotegerin, and Sclerostin as Potential Mediators of the Tophus-bone Erosion Relationship in Gout. J Rheumatol. 2016; 43(2):445-449. [36] HARRE U, DERER A, SCHORN C, et al. T cells as key players for bone destruction in gouty arthritis?. Arthritis Res Ther. 2011;13(6):135. [37] DALBETH N, SMITH T, NICOLSON B, et al. Enhanced osteoclastogenesis in patients with tophaceous gout: urate crystals promote osteoclast development through interactions with stromal cells. Arthritis Rheum. 2008;58(6):1854-1865. [38] CHOE JY, PARK KY, KIM SK. Monosodium Urate in the Presence of RANKL Promotes Osteoclast Formation through Activation of c-Jun N-Terminal Kinase. Mediators Inflamm. 2015;2015:597512. [39] MURAKAMI T, NAKAMINAMI Y, TAKAHATA Y, et al. Activation and Function of NLRP3 Inflammasome in Bone and Joint-Related Diseases. Int J Mol Sci. 2022; 23(10):5365. [40] GIAMARELLOS-BOURBOULIS EJ, MOUKTAROUDI M, BODAR E, et al. Crystalsof monosodium urate monohydrate enhance lipopolysaccharide-induced release of interleukin 1 beta by mononuclear cellsthrough a caspase 1-mediated process. Ann Rheum Dis. 2009;68(2):273-278. [41] MARTINON F, PÉTRILLI V, MAYOR A, et al. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440(7081):237-241. [42] CHEN CJ, SHI Y, HEARN A, et al. MyD88-dependent IL-1 receptorsignaling is essential for gouty inflammation stimulated by monosodium urate crystals. J Clin Invest. 2006;116(8):2262-2271. [43] SO A, DE SMEDT T, REVAZ S, et al. A pilot study of IL-1 inhibition by anakinra in acute gout. Arthritis Res Ther. 2007;9(2):R28. [44] SCHLESINGER N, ALTEN RE, BARDIN T, et al. Canakinumab for acute gouty arthritis in patients with limited treatment options: Results from two randomised, multicentre,active-controlled, double-blind trials and their initial extensions. Ann Rheum Dis. 2012;71(11):1839-1848. [45] DALBETH N, GOSLING AL, GAFFO A, et al. Gout. Lancet. 2021;397(10287):1843-1855. [46] NAREDO E, USON J, JIMÉNEZ-PALOP M, et al. Ultrasound-detected musculoskeletal urate crystal deposition: which joints and what findings should be assessed for diagnosing gout? Ann Rheum Dis. 2014;73(8):1522-1528. [47] KOTAKE S, UDAGAWA N, HAKODA M, et al. Activated human T cells directly induce osteoclastogenesis from human monocytes: possible role of T cells in bone destruction in rheumatoid arthritis patients. Arthritis Rheum. 2001;44(5):1003-1012. [48] TAO SS, CAO F, SAM NB, et al. Dickkopf-1 as a promising therapeutic target for autoimmune diseases. Clin Immunol. 2022;245:109156. [49] HUANG Y, LIU L, LIU A. Dickkopf-1: Current knowledge and related diseases. Life Sci. 2018;209:249-254. [50] 赵卫,高辉,朱佳鑫,等. 血清Dickkopf-1与原发性痛风性关节炎骨破坏的相关性[J].北京大学学报(医学版),2012,44(2):254-258. [51] GAVRIATOPOULOU M, DIMOPOULOS MA, CHRISTOULAS D, et al. Dickkopf-1: a suitable target for the management of myeloma bone disease. Expert Opin Ther Targets. 2009;13(7):839-848. [52] CHO YN, JEONG HS, PARK KJ, et al. Altered distribution and enhanced osteoclastogenesis of mucosal-associated invariant T cells in gouty arthritis. Rheumatology (Oxford). 2020;59(8):2124-2134. [53] CHUNG R, COOL JC, SCHERER MA, et al. Roles of neutrophil-mediated inflammatory response in the bony repair of injured growth plate cartilage in young rats. J Leukoc Biol. 2006;80(6):1272-1280. [54] GUERRA M, HALLS VS, SCHATTERNY J, et al. Protease FRET Reporters Targeting Neutrophil Extracellular Traps. J Am Chem Soc. 2020;142(48):20299-20305. [55] DANIEL C, LEPPKES M, MUÑOZ LE, et al. Extracellular DNA traps in inflammation, injury and healing. Nat Rev Nephrol. 2019;15(9):559-575. [56] DAI XJ, TAO JH, FANG X, et al. Changes of Treg/Th17 Ratio in Spleen of Acute Gouty Arthri-tis Rat Induced by MSU Crystals. Inflammation. 2018;41(5):1955-1964. [57] JIA E, LI Z, GENG H, et al. Neutrophil extracellular traps induce the bone erosion of gout. BMC Musculoskelet Disord. 2022;23(1):1128. [58] D HAYASHI R, YAMAOKA M, NISHIZAWA H, et al. Multiple Gouty Tophi with Bone Erosion and Destruction: A Report of an Early-onset Case in an Obese Patient. Intern Med. 2017;56(9):1071-1077. [59] MATSUO H, TAKADA T, ICHIDA K, et al. Common defects of ABCG2, a high-capacity urate exporter, cause gout: a function-based genetic analysis in a Japanese population. Sci Transl Med. 2009;1(5):5ra11. [60] NAKAYAMA A, MATSUO H, SHIMIZU T, et al. Common missense variant of monocarboxylate transporter 9 (MCT9/SLC16A9) gene is associated with renal overload gout, but not with all gout susceptibility. Hum Cell. 2013;26(4):133-136. [61] HAINER BL, MATHESON E, WILKES RT. Diagnosis, treatment, and prevention of gout. Am Fam Physician. 2014;90(12):831-836. [62] 王旭,罗冬平,茹彦海,等.从慢性肾脏病角度看高尿酸血症与痛风的指南更新要点[J].中国全科医学,2021,24(33):4191-4195. [63] FITZGERALD JD, DALBETH N, MIKULS T, et al. 2020 American College of Rheumatology Guideline for the Management of Gout. Arthritis Rheumatol. 2020;72(6):879-895. [64] HU S, TERKELTAUB R, SUN M, et al. Palpable tophi and more comorbidities associated with adherence to urate-lowering medical therapy in a Chinese gout cohort. Joint Bone Spine. 2022;89(6):105435. |
| [1] | Ma Shuwei, He Sheng, Han Bing, Zhang Liaoyun. Exosomes derived from mesenchymal stem cells in treatment of animals with acute liver failure: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1137-1142. |
| [2] | Zhang Kefan, Shi Hui. Research status and application prospect of cytokine therapy for osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(6): 961-967. |
| [3] | Zhang Ming, Wang Bin, Jia Fan, Chen Jie, Tang Wei. Application of brain-computer interface technology based on electroencephalogram in upper limb motor function rehabilitation of stroke patients [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 581-586. |
| [4] | He Yuanjie, Chen Yuheng, Zhao Yongchao, Wang Zhenglong. Progress in epigenetic regulation of vascular smooth muscle cell remodeling in the occurrence and development of aortic aneurysms [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 602-608. |
| [5] | Ma Sicong, Chen Jing, Li Yunqing. Functions and roles of connective tissue growth factor in nervous systems [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 615-620. |
| [6] | Yan Binghan, Li Zhichao, Su Hui, Xue Haipeng, Xu Zhanwang, Tan Guoqing. Mechanisms of traditional Chinese medicine monomers in the treatment of osteoarthritis by targeting autophagy [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 627-632. |
| [7] | Wang Xinyi, Xie Xianrui, Chen Yujie, Wang Xiaoyu, Xu Xiaoqing, Shen Yihong, Mo Xiumei. Electrospun nanofiber scaffolds for soft and hard tissue regeneration [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(3): 426-432. |
| [8] | Long Jundong, Shi Yehong, Wang Cheng, Chen Shijiu. Effects of different freezing techniques on the rejection of allogeneic vascular transplantation [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(3): 433-438. |
| [9] | Yang Jie, Hu Haolei, Li Shuo, Yue Wei, Xu Tao, Li Yi. Application of bio-inks for 3D printing in tissue repair and regenerative medicine [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(3): 445-451. |
| [10] | Long Yi, Yang Jiaming, Ye Hua, Zhong Yanbiao, Wang Maoyuan. Extracellular vesicles in sarcopenic obesity: roles and mechanisms [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(2): 315-320. |
| [11] | Chang Wanpeng, Zhang Zhongwen, Yang Yulin, Zi Yang, Yang Mengqi, Du Bingyu, Wang Nan, Yu Shaohong. Efficacy of rehabilitation exoskeleton robots on post-stroke lower limb motor dysfunction: a Meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(2): 321-328. |
| [12] | Dai Xinyu, Yan Jihong, Hua Lingjun, Zheng Xiaohong. Resistance exercise improves body composition in overweight and obese people: an umbrella review [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(2): 267-271. |
| [13] | Meng Zhicheng, Qiao Weiping, Zhao Yang, Liu Hongfei, Li Kaijie, Ma Bo. Effects of immune cells and related cytokines in the pathogenesis and treatment of osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(2): 280-287. |
| [14] | Long Qingxi, Zhang Pingshu, Liu Qing, Ou Ya, Zhang Lili, Yuan Xiaodong. Single-cell RNA sequencing reveals the heterogeneity of astrocytes [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(1): 139-146. |
| [15] | Nong Fuxiang, Jiang Zhixiong, Li Yinghao, Xu Wencong, Shi Zhilan, Luo Hui, Zhang Qinglang, Zhong Shuang, Tang Meiwen. Bone cement augmented proximal femoral nail antirotation for type A3.3 intertrochanteric femoral fracturalysis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(在线): 1-10. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||