[1] 黄燕靖,张明敏,周凡茹,等.早发性卵巢功能不全致病因素及机制研究进展[J].中西医结合研究,2022,14(1):48-51+55.
[2] SULLIVAN SD, SARREL PM, NELSON LM. Hormone replacement therapy in young women with primary ovarian insufficiency and early menopause. Fertil Steril. 2016;106(7):1588-1599.
[3] BOMPOULA MS, VALSAMAKIS G, NEOFYTOU S, et al. Demographic, clinical and hormonal characteristics of patients with premature ovarian insufficiency and those of early menopause: data from two tertiary premature ovarian insufficiency centers in Greece. Gynecol Endocrinol. 2020;36(8):693-697.
[4] US Preventive Services Task Force, GROSSMAN DC, CURRY SJ, et al. Hormone Therapy for the Primary Prevention of Chronic Conditions in Postmenopausal Women: US Preventive Services Task Force Recommendation Statement. JAMA. 2017;318(22):2224-2233.
[5] GUPTA S, LODHA P, KARTHICK MS, et al. Role of Autologous Bone Marrow-Derived Stem Cell Therapy for Follicular Recruitment in Premature Ovarian Insufficiency: Review of Literature and a Case Report of World’s First Baby with Ovarian Autologous Stem Cell Therapy in a Perimenopausal Woman of Age 45 Year. J Hum Reprod Sci. 2018;11(2):125-130.
[6] TESFAYE D, GEBREMEDHN S, SALILEW-WONDIM D, et al. MicroRNAs: tiny molecules with a significant role in mammalian follicular and oocyte development. Reproduction. 2018;155(3):R121-R135.
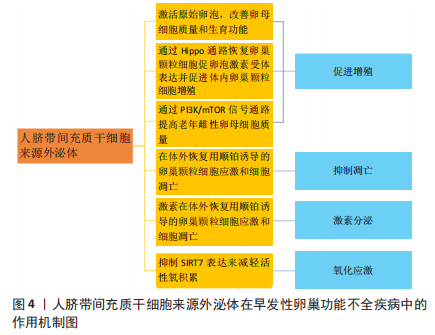
[7] DING C, ZHU L, SHEN H, et al. Exosomal miRNA-17-5p derived from human umbilical cord mesenchymal stem cells improves ovarian function in premature ovarian insufficiency by regulating SIRT7. Stem Cells. 2020;38(9):1137-1148.
[8] KOWAL J, TKACH M, THÉRY C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol. 2014;29:116-125.
[9] SALAS-HUETOS A, JAMES ER, ASTON KI, et al.The Expression of miRNAs in Human Ovaries, Oocytes, Extracellular Vesicles, and Early Embryos: A Systematic Review. Cells. 2019;8(12):1564.
[10] VLASSOV AV, MAGDALENO S, SETTERQUIST R, et al. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 2012;1820(7):940-948.
[11] KALLURI R, LEBLEU VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478):eaau6977.
[12] GALLO A, TANDON M, ALEVIZOS I, et al. The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS One. 2012;7(3):e30679.
[13] SUN D, ZHUANG X, ZHANG S, et al. Exosomes are endogenous nanoparticles that can deliver biological information between cells. Adv Drug Deliv Rev. 2013;65(3):342-347.
[14] ZHAO AG, SHAH K, CROMER B, et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles and Their Therapeutic Potential. Stem Cells Int. 2020;2020:8825771.
[15] FITZGERALD JB, GEORGE J, CHRISTENSON LK. Non-coding RNA in Ovarian Development and Disease. Adv Exp Med Biol. 2016;886:79-93.
[16] ZHANG S, HUANG B, SU P, et al. Concentrated exosomes from menstrual blood-derived stromal cells improves ovarian activity in a rat model of premature ovarian insufficiency. Stem Cell Res Ther. 2021;12(1):178.
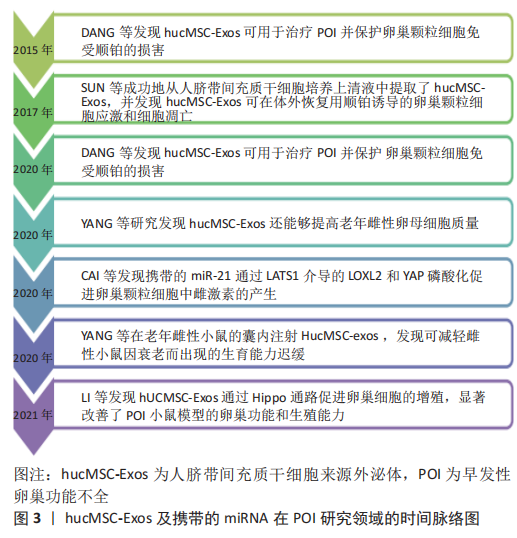
[17] DANG J, JIN Z, LIU X, et al. Human cord blood mononuclear cell transplantation for the treatment of premature ovarian failure in nude mice. Int J Clin Exp Med. 2015;8(3):4122-4127.
[18] LI Z, ZHANG M, ZHENG J, et al. Human Umbilical Cord Mesenchymal Stem Cell-Derived Exosomes Improve Ovarian Function and Proliferation of Premature Ovarian Insufficiency by Regulating the Hippo Signaling Pathway. Front Endocrinol (Lausanne). 2021;12:711902.
[19] MEHRI S, LEVI SETTI PE, GRECO K, et al. Correlation between follicular diameters and flushing versus no flushing on oocyte maturity, fertilization rate and embryo quality. J Assist Reprod Genet. 2014;31(1):73-77.
[20] YANG W, ZHANG J, XU B, et al. HucMSC-Derived Exosomes Mitigate the Age-Related Retardation of Fertility in Female Mice. Mol Ther. 2020;28(4):1200-1213.
[21] 杨玮杰. 人脐带间充质干细胞来源外泌体激活原始卵泡的研究[D].南京:南京医科大学,2018.
[22] DANG Y, WANG X, HAO Y, et al. MicroRNA-379-5p is associate with biochemical premature ovarian insufficiency through PARP1 and XRCC6. Cell Death Dis. 2018; 9(2):106.
[23] 张群. microRNA181a直接靶向acvr2a抑制小鼠卵巢颗粒细胞的增殖[D].南京:南京医科大学,2012.
[24] ZHANG X, DANG Y, LIU R, et al. MicroRNA-127-5p impairs function of granulosa cells via HMGB2 gene in premature ovarian insufficiency. J Cell Physiol. 2020; 235(11):8826-8838.
[25] 张新玥. MicroRNA-127-5p及环状RNA在早发性卵巢功能不全中的作用及机制研究[D].济南:山东大学,2021.
[26] SANTORO N. Mechanisms of premature ovarian failure. Ann Endocrinol (Paris). 2003;64(2):87-92.
[27] LIU M, QIU Y, XUE Z, et al. Small extracellular vesicles derived from embryonic stem cells restore ovarian function of premature ovarian failure through PI3K/AKT signaling pathway. Stem Cell Res Ther. 2020;11(1):3.
[28] SUN L, LI D, SONG K, et al. Exosomes derived from human umbilical cord mesenchymal stem cells protect against cisplatin-induced ovarian granulosa cell stress and apoptosis in vitro. Sci Rep. 2017;7(1):2552.
[29] XIAO GY, CHENG CC, CHIANG YS, et al. Exosomal miR-10a derived from amniotic fluid stem cells preserves ovarian follicles after chemotherapy. Sci Rep. 2016;6:23120.
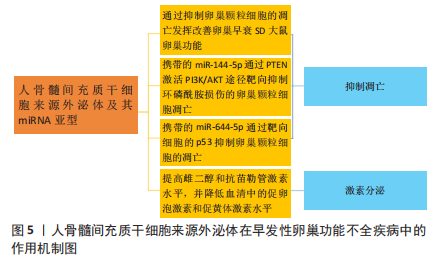
[30] SUN B, MA Y, WANG F, et al. miR-644-5p carried by bone mesenchymal stem cell-derived exosomes targets regulation of p53 to inhibit ovarian granulosa cell apoptosis. Stem Cell Res Ther. 2019;10(1):360.
[31] 周政. 骨髓间充质干细胞外泌体对SD大鼠早衰卵巢影响机制研究[D].锦州:锦州医科大学,2018.
[32] ZHANG Q, SUN J, HUANG Y, et al. Human Amniotic Epithelial Cell-Derived Exosomes Restore Ovarian Function by Transferring MicroRNAs against Apoptosis. Mol Ther Nucleic Acids. 2019;16:407-418.
[33] 赵玮. 脂肪干细胞来源的外泌体对大鼠卵巢早衰的作用及机制研究[D].上海:中国人民解放军海军军医大学,2020.
[34] FU X, HE Y, WANG X, et al. Overexpression of miR-21 in stem cells improves ovarian structure and function in rats with chemotherapy-induced ovarian damage by targeting PDCD4 and PTEN to inhibit granulosa cell apoptosis. Stem Cell Res Ther. 2017;8(1):187.
[35] 李欣然,何援利,王雪峰,等.miR-21调控PTEN及PDCD4基因治疗化疗性卵巢早衰[J].现代妇产科进展,2017,26(9):661-665.
[36] YANG M, LIN L, SHA C, et al. Bone marrow mesenchymal stem cell-derived exosomal miR-144-5p improves rat ovarian function after chemotherapy-induced ovarian failure by targeting PTEN. Lab Invest. 2020;100(3):342-352.
[37] HUANG B, LU J, DING C, et al. Exosomes derived from human adipose mesenchymal stem cells improve ovary function of premature ovarian insufficiency by targeting SMAD. Stem Cell Res Ther. 2018;9(1):216.
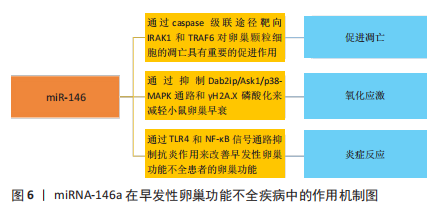
[38] CHEN X, XIE M, LIU D, et al. Downregulation of microRNA 146a inhibits ovarian granulosa cell apoptosis by simultaneously targeting interleukin 1 receptor associated kinase and tumor necrosis factor receptor associated factor 6. Mol Med Rep. 2015;12(4):5155-5162.
[39] ZHANG X, ZHANG R, HAO J, et al. miRNA-122-5p in POI ovarian-derived exosomes promotes granulosa cell apoptosis by regulating BCL9. Cancer Med. 2022;11(12):2414-2426.
[40] ZHAO H, GU W, PAN W, et al. miR-483-5p aggravates cisplatin-induced premature ovarian insufficiency in rats by targeting FKBP4. Nan Fang Yi Ke Da Xue Xue Bao. 2021;41(6):801-810.
[41] 赵茴茴,古文清,潘文斌,等.miR-483-5p通过靶向FKBP4加重顺铂诱导的小鼠卵巢损伤[J].南方医科大学学报,2021,41(6):801-810.
[42] YANG X, ZHOU Y, PENG S, et al. Differentially expressed plasma microRNAs in premature ovarian failure patients and the potential regulatory function of mir-23a in granulosa cell apoptosis. Reproduction. 2012;144(2):235-244.
[43] 董小敏,李睿,杨进,等.miR-204-5p对卵巢早衰大鼠卵巢GCs凋亡的调控机制[J].西部医学,2021,33(5):636-643+649.
[44] 张群. microRNA-181a调控卵巢颗粒细胞凋亡的机制研究[D].南京:南京大学,2015.
[45] SIROTKIN AV, OVCHARENKO D, GROSSMANN R, et al. Identification of microRNAs controlling human ovarian cell steroidogenesis via a genome-scale screen. J Cell Physiol. 2009;219(2):415-420.
[46] DAI A, SUN H, FANG T, et al. MicroRNA-133b stimulates ovarian estradiol synthesis by targeting Foxl2. FEBS Lett. 2013;587(15):2474-2482.
[47] CAI JH, SUN YT, BAO S. HucMSCs-exosomes containing miR-21 promoted estrogen production in ovarian granulosa cells via LATS1-mediated phosphorylation of LOXL2 and YAP. Gen Comp Endocrinol. 2022;321-322:114015.
[48] 徐一江. MiR-204-5p/lnc-GULP1-2:1/COL3A1协同调控卵巢早衰发生的机制研究[D].郑州:郑州大学,2017.
[49] LIM J, ALI S, LIAO LS, et al. Antioxidant supplementation partially rescues accelerated ovarian follicle loss, but not oocyte quality, of glutathione-deficient mice†. Biol Reprod. 2020;102(5):1065-1079.
[50] DING C, QIAN C, HOU S, et al. Exosomal miRNA-320a Is Released from hAMSCs and Regulates SIRT4 to Prevent Reactive Oxygen Species Generation in POI. Mol Ther Nucleic Acids. 2020;21:37-50.
[51] LIU T, LIU Y, HUANG Y, et al. miR-15b induces premature ovarian failure in mice via inhibition of α-Klotho expression in ovarian granulosa cells. Free Radic Biol Med. 2019;141:383-392.
[52] LIU T, LIN J, CHEN C, et al. MicroRNA-146b-5p overexpression attenuates premature ovarian failure in mice by inhibiting the Dab2ip/Ask1/p38-Mapk pathway and γH2A.X phosphorylation. Cell Prolif. 2021;54(1):e12954.
[53] HE F, LIU Y, LI T, et al. MicroRNA-146 attenuates lipopolysaccharide induced ovarian dysfunction by inhibiting the TLR4/NF-κB signaling pathway. Bioengineered. 2022;13(5):11611-11623.
[54] KOUREMBANAS S. Exosomes: vehicles of intercellular signaling, biomarkers, and vectors of cell therapy. Annu Rev Physiol. 2015;77:13-27.
[55] DA SILVEIRA JC, WINGER QA, BOUMA GJ, et al. Effects of age on follicular fluid exosomal microRNAs and granulosa cell transforming growth factor-β signalling during follicle development in the mare. Reprod Fertil Dev. 2015;27(6):897-905.
[56] ZHOU K, JIANG J, WU J, et al. Electroacupuncture modulates reproductive hormone levels in patients with primary ovarian insufficiency: results from a prospective observational study. Evid Based Complement Alternat Med. 2013; 2013:657234.
[57] MA S, WU J, FENG Y, et al. Elevated estrogen receptor expression in hypothalamic preoptic area decreased by electroacupuncture in ovariectomized rats. Neurosci Lett. 2011;494(2):109-113.
[58] FENG Y, JOHANSSON J, SHAO R, et al. Hypothalamic neuroendocrine functions in rats with dihydrotestosterone-induced polycystic ovary syndrome: effects of low-frequency electro-acupuncture. PLoS One. 2009;4(8):e6638.
[59] 李柠岑,李明月,李牧洋,等.外泌体在针刺靶向趋病中的作用探讨[J].中华中医药杂志,2021,36(10):6222-6224.
[60] 李牧洋,王婷婷,陈波,等.电针足三里-环跳穴对不同状态大鼠血清外泌体表达的影响[J].陕西中医,2019,40(2):139-142.
[61] 班维固,滕秀英,齐辉.针灸疗法调控microRNA在基础实验中应用的研究进展[J].中医药导报,2021,27(7):138-142+147.
[62] 李璐,孙博,孙莹璞.间充质干细胞来源外泌体治疗原发性卵巢功能不全机制研究进展[J].中华生殖与避孕杂志,2021,41(12):1091-1095.
[63] 张秀娟,张瑞红,郝晶, 等.外泌体及其miRNA治疗化疗所致早发性卵巢功能不全的研究进展[J].现代妇产科进展,2021,30(5):389-391. |