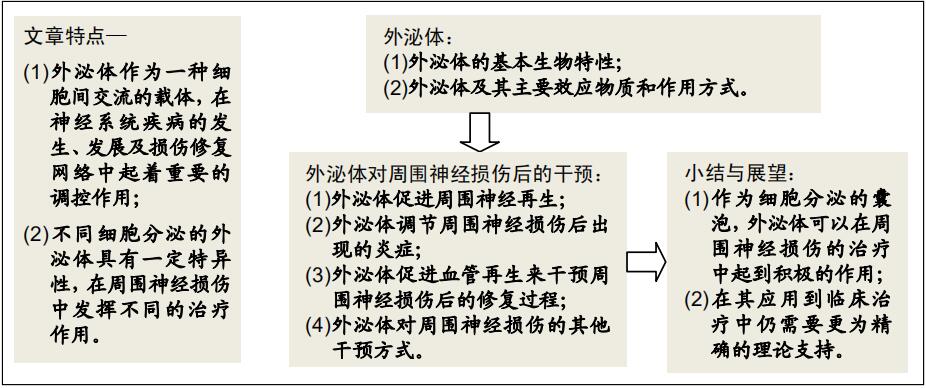
|
[1] SAFA B, BUNCKE G. Autograft Substitutes: Conduits and Processed Nerve Allografts. Hand Clin. 2016;32(2):127-140.
[2] NAWAZ M, FATIMA F, VALLABHANENI KC, et al. Extracellular Vesicles: Evolving Factors in Stem Cell Biology. Stem Cells Int. 2016;2016:1073140.
[3] RAPOSO G, STOORVOGEL W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373-383.
[4] HARDING C, HEUSER J, STAHL P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983;97(2):329-339.
[5] PAN BT, TENG K, WU C, et al. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol.1985;101(3):942-948.
[6] ZHANG Y, CHOPP M, LIU XS, et al. Exosomes Derived from Mesenchymal Stromal Cells Promote Axonal Growth of Cortical Neurons. Mol Neurobiol. 2017;54(4):2659-2673.
[7] THÉRY C, ZITVOGEL L, AMIGORENA S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002; 2(8):569-579.
[8] JING H, HE X, ZHENG J. Exosomes and regenerative medicine: state of the art and perspectives. Transl Res. 2018;196:1-16.
[9] SIMBARI F, MCCASKILL J, COAKLEY G, et al. Plasmalogen enrichment in exosomes secreted by a nematode parasite versus those derived from its mouse host: implications for exosome stability and biology. J Extracell Vesicles. 2016;5:30741.
[10] BOBRIE A, COLOMBO M, RAPOSO G, et al. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic. 2011;12(12):1659-1668.
[11] MATHIVANAN S, SIMPSON RJ. ExoCarta: A compendium of exosomal proteins and RNA. Proteomics. 2009;9(21):4997-5000.
[12] HA D, YANG N, NADITHE V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm Sin B. 2016;6(4):287-296.
[13] WAHLGREN J, STATELLO L, SKOGBERG G, et al. Delivery of Small Interfering RNAs to Cells via Exosomes. Methods Mol Biol. 2016;1364:105-125.
[14] 覃思华,郑磊.外泌体蛋白质组学研究进展[J].临床检验杂志, 2016, 34(12):927-930.
[15] MITTELBRUNN M, SÁNCHEZ-MADRID F. Intercellular communication: diverse structures for exchange of genetic information. Nat Rev Mol Cell Biol. 2012;13(5):328-335.
[16] LOTVALL J, VALADI H. Cell to cell signalling via exosomes through esRNA. Cell Adh Migr. 2007;1(3):156-158.
[17] BARTEL DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215-233.
[18] ENGELS BM, HUTVAGNER G. Principles and effects of microRNA-mediated post-transcriptional gene regulation. Oncogene. 2006;25(46):6163-6169.
[19] GUO H, INGOLIA NT, WEISSMAN JS, et al. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010;466(7308):835-840.
[20] BARTEL DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281-297.
[21] WANG GG, KONZE KD, TAO J. Polycomb genes, miRNA, and their deregulation in B-cell malignancies. Blood. 2015;125(8): 1217-1225.
[22] ELDH M, EKSTRÖM K, VALADI H, et al. Exosomes communicate protective messages during oxidative stress; possible role of exosomal shuttle RNA. PLoS One. 2010;5(12):e15353.
[23] KOSAKA N, IGUCHI H, YOSHIOKA Y, et al. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 2010;285(23):17442-17452.
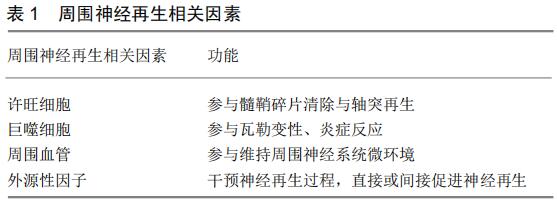
[24] GLENN TD, TALBOT WS. Signals regulating myelination in peripheral nerves and the Schwann cell response to injury. Curr Opin Neurobiol. 2013;23(6):1041-1048.
[25] LOPEZ-VERRILLI MA, PICOU F, COURT FA. Schwann cell-derived exosomes enhance axonal regeneration in the peripheral nervous system. Glia. 2013;61(11):1795-1806.
[26] LOPEZ-VERRILLI MA, COURT FA. Transfer of vesicles from schwann cells to axons: a novel mechanism of communication in the peripheral nervous system. Front Physiol. 2012;3:205.
[27] LI S, ZHANG R, YUAN Y, et al. MiR-340 Regulates Fibrinolysis and Axon Regrowth Following Sciatic Nerve Injury. Mol Neurobiol. 2017;54(6):4379-4389.
[28] LOPEZ-LEAL R, COURT FA. Schwann Cell Exosomes Mediate Neuron-Glia Communication and Enhance Axonal Regeneration. Cell Mol Neurobiol. 2016;36(3):429-436.
[29] LOPEZ-VERRILLI MA, COURT FA. Exosomes: mediators of communication in eukaryotes. Biol Res. 2013;46(1):5-11.
[30] GEHLER S, GALLO G, VEIEN E, et al. p75 neurotrophin receptor signaling regulates growth cone filopodial dynamics through modulating RhoA activity. J Neurosci. 2004;24(18):4363-4372.
[31] TIMMERS L, LIM SK, ARSLAN F, et al. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res. 2007;1(2):129-137.
[32] BIAN S, ZHANG L, DUAN L, et al. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J Mol Med (Berl). 2014;92(4):387-397.
[33] XIN H, LI Y, CUI Y, et al. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab. 2013;33(11):1711-1715.
[34] ZHANG Y, CHOPP M, MENG Y, et al. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J Neurosurg. 2015;122(4):856-867.
[35] LOPEZ-VERRILLI MA, CAVIEDES A, CABRERA A, et al. Mesenchymal stem cell-derived exosomes from different sources selectively promote neuritic outgrowth. Neuroscience. 2016;320: 129-139.
[36] MEAD B, TOMAREV S. Bone Marrow-Derived Mesenchymal Stem Cells-Derived Exosomes Promote Survival of Retinal Ganglion Cells Through miRNA-Dependent Mechanisms. Stem Cells Transl Med. 2017;6(4):1273-1285.
[37] RAO F, ZHANG D, FANG T, et al. Exosomes from Human Gingiva-Derived Mesenchymal Stem Cells Combined with Biodegradable Chitin Conduits Promote Rat Sciatic Nerve Regeneration. Stem Cells Int. 2019;2019:2546367.
[38] BUCAN V, VASLAITIS D, PECK CT, et al. Effect of Exosomes from Rat Adipose-Derived Mesenchymal Stem Cells on Neurite Outgrowth and Sciatic Nerve Regeneration After Crush Injury. Mol Neurobiol. 2019;56(3):1812-1824.
[39] LIU CY, YIN G, SUN YD, et al. Effect of exosomes from adipose-derived stem cells on the apoptosis of Schwann cells in peripheral nerve injury. CNS Neurosci Ther. 2019 Jul 6. doi: 10.1111/cns.13187.
[40] CHEN J, REN S, DUSCHER D, et al. Exosomes from human adipose-derived stem cells promote sciatic nerve regeneration via optimizing Schwann cell function. J Cell Physiol. 2019;234(12): 23097-23110.
[41] KALRA H, SIMPSON RJ, JI H, et al. Vesiclepedia: a compendium for extracellular vesicles with continuous community annotation. PLoS Biol. 2012;10(12):e1001450.
[42] HESSVIK NP, LLORENTE A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci. 2018;75(2):193-208.
[43] BOSSE F. Extrinsic cellular and molecular mediators of peripheral axonal regeneration. Cell Tissue Res. 2012;349(1):5-14.
[44] KRÄMER-ALBERS EM, BRETZ N, TENZER S, et al. Oligodendrocytes secrete exosomes containing major myelin and stress-protective proteins: Trophic support for axons? Proteomics Clin Appl. 2007;1(11):1446-1461.
[45] ZHAO Z, ZLOKOVIC BV. Remote control of BBB: A tale of exosomes and microRNA. Cell Res. 2017;27(7):849-850.
[46] CHAN BD, WONG WY, LEE MM, et al. Exosomes in Inflammation and Inflammatory Disease. Proteomics. 2019;19(8):e1800149.
[47] SOMMER C, LEINDERS M, ÜÇEYLER N. Inflammation in the pathophysiology of neuropathic pain. Pain. 2018;159(3):595-602.
[48] WEI Z, FAN B, DING H, et al. Proteomics analysis of Schwann cell-derived exosomes: a novel therapeutic strategy for central nervous system injury. Mol Cell Biochem. 2019;457(1-2):51-59.
[49] GAUDET AD, LEUNG M, POIRIER F, et al. A role for galectin-1 in the immune response to peripheral nerve injury. Exp Neurol. 2009; 220(2):320-327.
[50] 边洪琳,季爱玉,熊乐.周围神经损伤后背根神经节神经元及M1、M2型巨噬细胞变化[J].青岛大学医学院学报, 2016,52(5):525-528.
[51] TI D, HAO H, TONG C, et al. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J Transl Med. 2015;13:308.
[52] SUN G, LI G, LI D, et al. hucMSC derived exosomes promote functional recovery in spinal cord injury mice via attenuating inflammation. Mater Sci Eng C Mater Biol Appl. 2018;89:194-204.
[53] TAGANOV KD, BOLDIN MP, CHANG KJ, et al. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A. 2006;103(33): 12481-12486.
[54] KIM SH, LECHMAN ER, BIANCO N, et al. Exosomes derived from IL-10-treated dendritic cells can suppress inflammation and collagen-induced arthritis. J Immunol. 2005;174(10):6440-6448.
[55] 钱杰,冯兴梅.牙髓干细胞及其外泌体的应用新进展[J].交通医学, 2018,32(2):117-120.
[56] SHIH T, LINDLEY C. Bevacizumab: an angiogenesis inhibitor for the treatment of solid malignancies. Clin Ther. 2006;28(11): 1779-1802.
[57] ZHANG B, WU X, ZHANG X, et al. Human umbilical cord mesenchymal stem cell exosomes enhance angiogenesis through the Wnt4/β-catenin pathway. Stem Cells Transl Med. 2015;4(5): 513-522.
[58] JOHNSON TK, ZHAO L, ZHU D, et al. Exosomes derived from induced vascular progenitor cells promote angiogenesis in vitro and in an in vivo rat hindlimb ischemia model. Am J Physiol Heart Circ Physiol. 2019;317(4):H765-H776.
[59] SHIUE SJ, RAU RH, SHIUE HS, et al. Mesenchymal stem cell exosomes as a cell-free therapy for nerve injury-induced pain in rats. Pain. 2019;160(1):210-223.
[60] 黎博,魏红艳,杨焰,等.神经干细胞外泌体对低氧所致神经元凋亡的抑制作用[J].中国病理生理杂志,2018,34(4):717-722,728.
|