中国组织工程研究 ›› 2019, Vol. 23 ›› Issue (22): 3542-3548.doi: 10.3969/j.issn.2095-4344.1738
• 材料生物相容性 material biocompatibility • 上一篇 下一篇
去端肽Ⅰ型胶原蛋白的免疫原性研究
张自强1,张以河1,安 琪1,徐荣荣2,李 欢2,王恩博3
- 1非金属矿物与固废资源材料化利用北京市重点实验室,中国地质大学(北京)材料科学与工程学院,北京市 102200;2北京湃生生物科技有限公司,北京市 102200;3北京大学口腔医学院口腔颌面外科,北京市 100081
Study on immunogenicity of type I atelocollagen
Zhang Ziqiang1, Zhang Yihe1, An Qi1, Xu Rongrong2, Li Huan2, Wang Enbo3
- 1Beijing Key Laboratory of Material Utilization of Non-metallic Minerals and Solid Waste Resources, School of Materials Science and Technology, China University of Geosciences, Beijing 102200, China; 2Beijing Peisheng Biotech Co., Ltd., Beijing 102200, China; 3Department of Oral and Maxillofacial Surgery, Peking University Hospital of Stomatology, Beijing 100081, China
摘要:
文章快速阅读:
.jpg)
文题释义:
端肽:Ⅰ型胶原蛋白由2条α1多肽链一条α2多肽链组成的三维螺旋结构,核心为不间断重复Gly-X-Y三肽,两侧为球状域前肽,又叫“端肽”,这2个区域是由短的、非螺旋氨基酸序列所组成,是胶原的主要免疫原性位点,它可在提取胶原时被选择性的水解或去除而失活。
α-Gal抗原:是动物组织或器官引起免疫排斥反应的主要靶抗原,是一种含有半乳糖基的糖蛋白或糖脂类抗原物质,人体内存在与Gal抗原反应的特异性IgG天然Gal抗体的存在,导致动物组织或动物源性生物材料移植后免疫排斥反应的发生。
背景:Ⅰ型胶原蛋白的免疫原性主要分布在分子链的端肽区域,可在胶原蛋白提取过程中通过水解或是去除而使其失活,制备去端肽Ⅰ型胶原蛋白,降低其免疫原性。
目的:评价去端肽Ⅰ型胶原蛋白的免疫原性。
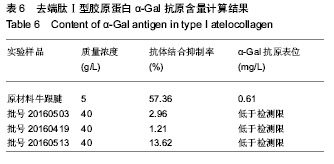
方法:采用酶切、盐析、透析工艺制备去端肽Ⅰ型胶原蛋白,通过考马斯亮蓝对牛血清白蛋白染色极限的分析,鉴定去端肽Ⅰ型胶原蛋白的纯度,依据YY/T 0606.25-2014中规定的方法,利用Quant-IT PicoGreen dsDNA Reagent and Kits试剂盒测定去端肽Ⅰ型胶原蛋白的DNA残留量,利用ELISA试剂盒分析去端肽Ⅰ型胶原蛋白的端肽去除效果,依据YY/T 1561-2017标准检测去端肽Ⅰ型胶原蛋白的α-Gal抗原含量。
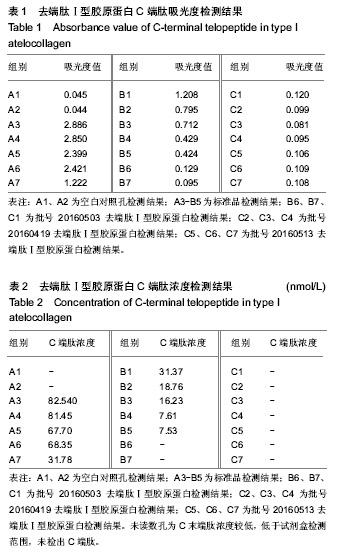
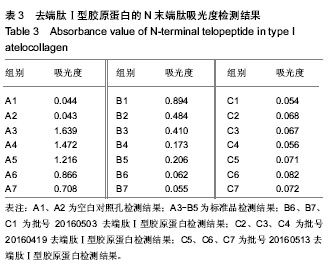
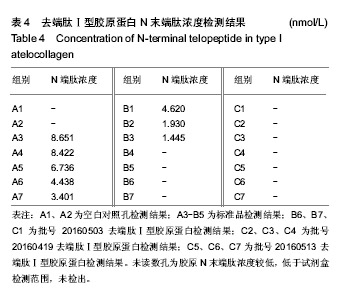
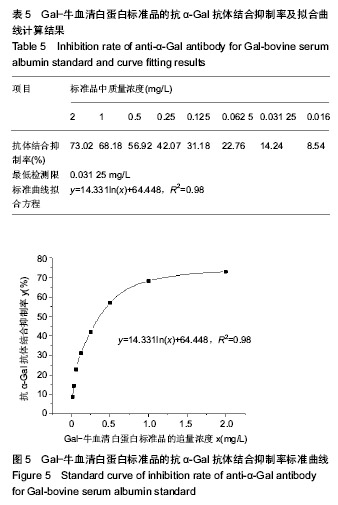
结果与结论:去端肽Ⅰ型胶原蛋白的纯度可达99.8%,DNA残留量为4 µg/g,C、N末端肽浓度低于试剂盒检测限,α-Gal抗原含量低于检测限。通过酶切、盐析、透析工艺制备的去端肽Ⅰ型胶原蛋白,可显著去除天然胶原蛋白的免疫原性根源,有效控制胶原蛋白临床应用的免疫原性风险。
中图分类号:

.jpg)
.jpg)
.jpg)