中国组织工程研究 ›› 2015, Vol. 19 ›› Issue (7): 996-1002.doi: 10.3969/j.issn.2095-4344.2015.07.003
• 骨组织构建 bone tissue construction • 上一篇 下一篇
骨性关节炎与类风湿关节炎的股骨近端骨结构三维微观结构分析
王佰亮1, Ming Ding2, Søren Overgaard3
1卫生部中日友好医院关节外科,北京市 100029;南丹麦大学欧登塞大学医院,2骨科实验室,3骨科,欧登塞,丹麦
-
出版日期:2015-02-12发布日期:2015-02-12 -
通讯作者:王佰亮,卫生部中日友好医院关节外科,北京市 100029 -
作者简介:王佰亮,男,1978年生,山东省潍坊市人,汉族, 2007年北京协和医学院毕业,博士,副主任医师。 -
基金资助:中日友好医院青年课题基金(2013-QN-19)
Three-dimensional microarchitecture of the proximal femur in osteoarthritis and rheumatoid arthritis
- 1 Department of Joint Surgery, China-Japan Friendship Hospital, Beijing 100029, China2 The Orthopaedic Research Laboratory, Department of Orthopaedic Surgery, Odense University Hospital, University of Southern Denmark, Odense, Denmark3 Department of Orthopaedic Surgery, Odense University Hospital, and Clinical Institute, University of Southern Denmark, Odense, Denmark
-
Online:2015-02-12Published:2015-02-12 -
Contact:Wang Bai-liang, Department of Joint Surgery, China-Japan Friendship Hospital, Beijing 100029, China -
About author:Wang Bai-liang, M.D., Associate chief physician, Department of Joint Surgery, China-Japan Friendship Hospital, Beijing 100029, China -
Supported by:the Youth Foundation of China-Japan Friendship Hospital, No. 2013-QN-19
摘要:
背景:人工关节假体松动的原因除了手术技术、假体设计及其产生的磨损颗粒等因素以外,假体周围的骨质量也起着极为重要的作用。骨微观结构是影响骨质量主要因素,应用技术分析组织微观结构目前已初步用于各种骨骼疾病的研究。 目的:应用micro-CT技术,观察类风湿性关节炎与骨性关节炎股骨近端骨组织性能变化,对比研究两者之间的骨微观结构差异。 方法:收集原发性骨关节病和类风湿性关节炎患者因行全髋关节置换而切除的股骨头(骨性关节炎10个,类风湿关节炎7个)。根据每个股骨头标本X射线上主要骨小梁方向,取材并制作来自于股骨颈骨组织的10 mm标本,然后进行micro-CT扫描,数据转换成3D图像数据后,分析两组之间皮质骨、松质骨以及股骨颈整体的骨微结构参数,做统计学处理。 结果与结论:骨性关节炎与类风湿性关节炎的股骨颈骨组织的微结构(皮质骨、松质骨以及整体)差异无显著性意义;两种疾病的股骨颈骨组织的整体微结构特点与松质骨结构特点相近。与类风湿患者相比,骨关节炎患者骨小梁的连接性密度丢失较多,皮质骨的骨小梁定向分布程度升高,而松质骨和整体的骨小梁定向分布程度降低。结果证实,骨性关节炎与类风湿性关节炎的皮质骨、松质骨以及整体股骨颈骨组织的微结构无显著差异,这提示两种疾病的股骨颈整体的微结构退变是相同的。
中国组织工程研究杂志出版内容重点:组织构建;骨细胞;软骨细胞;细胞培养;成纤维细胞;血管内皮细胞;骨质疏松;组织工程
中图分类号:
引用本文
王佰亮, Ming Ding, Søren Overgaard. 骨性关节炎与类风湿关节炎的股骨近端骨结构三维微观结构分析[J]. 中国组织工程研究, 2015, 19(7): 996-1002.
Wang Bai-liang, Ming Ding, Søren Overgaard. Three-dimensional microarchitecture of the proximal femur in osteoarthritis and rheumatoid arthritis[J]. Chinese Journal of Tissue Engineering Research, 2015, 19(7): 996-1002.
Difference in the three-dimensional microstructure of femoral neck specimens between osteoarthritis and rheumatoid arthritis groups
All the data were not distributed normally. Analyses of variation in the microstructural properties of different regions in the two groups are summarized in Table 1.
The patients with osteoarthritis were older than those with rheumatoid arthritis, but there was no significant difference. The entire cortical bone of the femoral neck in patients with rheumatoid arthritis had higher BV/TV, Co.Th*, Co.N* and Co.CD, lower Co.Sp, Co.DA and CSA than those with osteoarthritis, but there was no significant difference between the two groups. The entire trabecular bone in the rheumatoid arthritis group had higher BV/TV, Tb.N*, Tb.CD, Tb.DA and CSA, lower Tb.Th*, Tb.Sp than that in the osteoarthritis group, but there was no significant difference between the two groups. Properties of the femoral neck were similar to those of the trabecular bone. This decrease in bone volume in the osteoarthritis group resulted from the decreased amount of cortical and trabecular bone tissues as well as a consequent increase in dispersion degree. The osteoarthritis group was also characterized by a loss of the connectivity, an increase in DA for the cortical bone, but a decrease in DA for the cancellous bone and the entire trabecular bone when compared to the rheumatoid arthritis group.
Relations between the microstructural parameters of femoral neck specimens in osteoarthritis and rheumatoid arthritis groups
Among all the parameters, BV/TV had the strong correlation with Th*, Sp, SMI, and N* for the entire femoral head, cortical and trabecular bone in both osteoarthritis and rheumatoid arthritis groups. For all the three regions of rheumatoid arthritis and osteoarthritis patients, we found a BV/TV-related increase in Th* and a BV/TV-related decrease in Sp and SMI. But for the correlation between BV/TV and N*, there was no consistent tendency in the three regions of osteoarthritis and rheumatoid arthritis groups. The significant correlations between Sp and N* were also found in the three regions of osteoarthritis and rheumatoid arthritis groups.
| [1]Sun SS, Ma HL, Liu CL. Difference in femoral head and neck material properties between osteoarthritis and osteoporosis. Clin Biomech. 2008;23:S39-S47.[2]Issever AS, Burghardt A, Patel V, et al. A micro-computed tomography study of the trabecular bone structure in the femoral head. J Musculoskelet Neuronal Interact. 2003; 3(2):176-184. [3]Radin EL, Rose RM. Role of subchondral bone in the initiation and progression of cartilage damage. Clin Orthop. 1986;213:34-40. [4]Li BH, Aspden RM. Mechanical and material properties of the subchondral bone plate from the femoral head of patients with osteoarthritis or osteoporosis. Ann Rheum Dis. 1997;56:247-254. [5]Ding M, Christian Danielsen C, Hvid I. Bone density does not reflect mechanical properties in early-stage arthrosis. Acta Orthop Scand. 2001;72(2):181-185. [6]Dieppe P, Cushnaghan J, Young P, et al. Prediction of the progression of joint space narrowing in osteoarthritis of the knee by bone scintigraphy. Ann Rheum Dis. 1993;52: 557-563. [7]Chappard C, Peyrin F, Bonnassie A, et al. Subchondral bone micro-architectural alterations in osteoarthritis: a synchrotron micro-computed tomography study. Osteoarthritis Cartilage. 2006;14(3):215-223.[8]Cooper C, Cook PL, Osmond C, et al. Osteoarthritis of the hip and osteoporosis of the proximal femur. Ann Rheum Dis. 1991;50:540-542. [9]Dequeker J, Johnell O. Osteoathritis protects against femoral neck fracture: the MEDOS study experience. Bone. 1993;14:S51-S56. [10]Verstraeten A, Van Ermen H, Haghebaert G, et al. Osteoarthrosis retards the development of osteoporosis. Observation of the coexistence of osteoarthrosis and osteoporosis. Clin Orthop. 1991;264:169-177. [11]Gong H, Zhang M, Yeung HY, et al. Regional variations in microstructural properties of vertebral trabeculae with aging. J Bone Miner Metab. 2005;23:174-180. [12]Inaba M, Nagata M, Goto H, et al. Preferential reductions of paraarticular trabecular bone component in ultradistal radius and of calcaneus ultrasonography in early-stage rheumatoid arthritis. Osteoporos Int. 2003;14(8):683-687. [13]Korczowska I, Olewicz-Gawlik A, Trefler J, et al. Does low-dose and short-term glucocorticoids treatment increase the risk of osteoporosis in rheumatoid arthritis female patients? Clin Rheumatol. 2008;27(5):565-572. [14]Böttcher J, Pfeil A, Mentzel H, et al. Peripheral bone status in rheumatoid arthritis evaluated by digital X-ray radiogrammetry and compared with multisite quantitative ultrasound. Calcif Tissue Int. 2006;78(1):25-34. [15]Enokida M, Yamasaki D, Okano T, et al. Bone mass changes of tibial and vertebral bones in young and adult rats with collagen-induced arthritis. Bone. 2001;28(1): 87-93. [16]Le Corroller T, Pithioux M, Chaari F, et al. Bone texture analysis is correlated with three-dimensional microarchitecture and mechanical properties of trabecular bone in osteoporotic femurs. J Bone Miner Metab. 2013; 31(1):82-88. [17]Ollivier M, Le Corroller T, Blanc G, et al. Radiographic bone texture analysis is correlated with 3D microarchitecture in the femoral head, and improves the estimation of the femoral neck fracture risk when combined with bone mineral density. Eur J Radiol. 2013; 82(9):1494-1498. [18]Deodhar AA, Woolf AD. Bone mass measurement and bone metabolism in rheumatoid arthritis: a review. Br J Rheumatol. 1996;35(4):309-322. [19]Homminga J, Van-Rietbergen B, Lochmuller EM, et al. The osteoporotic vertebral structure is well adapted to the loads of daily life, but not to infrequent ‘‘error’’ loads. Bone. 2004; 34: 510-516. [20]Blain H, Chavassieux P, Portero-Muzy N, et al. Cortical and trabecular bone distribution in the femoral neck in osteoporosis and osteoarthritis. Bone. 2008;43(5):862-868. [21]Mayhew PM, Thomas CD, Clement JG, et al. Relation between age, femoral neck cortical stability, and hip fracture risk. Lancet. 2005;366:129-135. [22]Basso T, Klaksvik J, Syversen U, et al. A biomechanical comparison of composite femurs and cadaver femurs used in experiments on operated hip fractures. J Biomech. 2014; 18;47(16):3898-3902. [23]Bouxsein ML, Fajardo RF. Cortical stability of the femoral neck and hip fracture risk. Lancet. 2005;366:1532-1534.[24]Rivadeneira F, Zillikens MC. Femoral neck BMD is a strong predictor of hip fracture susceptibility in elderly men and women because it detects cortical bone instability: the rotterdam study. J Bone Miner Res. 2007;22:1781-1790. [25]Fazzalari NL, Parkinson IH. Femoral trabecular bone of osteoarthritic and normal subjects in an age and sex matched group. Osteoarth Cart. 1998;6:377-382. [26]Fazzalari NL, Moore RJ, Manthey BA, et al. Comparative study of iliac crest and subchondral femoral bone in osteoarthritis patients. Bone. 1992;13:331-335. [27]Hildebrand T, Rüegsegger P. Quantification of bone microarchitecture with the structure model index. Comput Methods Biomech Biomed Engin. 1997;1(1):15-23. [28]Odgaard A. Three-dimensional methods for quantification of cancellous bone architecture. Bone. 1997;20(4):315-328. [29]Tanck E, Bakker AD, Kregting S, et al. Predictive value of femoral head heterogeneity for fracture risk. Bone. 2009; 44(4):590-595. [30]Duque G, Troen BR. Understanding the mechanisms of senile osteoporosis: new facts for a major geriatric syndrome. J Am Geriatr Soc. 2008;56:935-941. [31]Bell KL, Loveridge N, Power J et al. Structure of the femoral neck in hip fracture: Cortical bone loss in the inferoanterior to superoposterior axis. J Bone Miner Res 1999;14: 111-119. [32]Lotz JC, Cheal EJ, Hayes WC. Stress distribution within the proximal femur during fait and falls: implications for osteoporotic fracture. Osteoporos Int. 1995;5:252-261. [33]Bell KL, Loveridge N, Power J, et al. Regional differences in cortical porosity in the fractured femoral neck. Bone. 1999; 24:57-64. [34]Lane NE, Pressman AR, Star VL, et al. Rheumatoid arthritis and bone mineral density in elderly women. The study of osteoporotic fractures research group. J Bone Miner Res. 1995;10(2):257-263. [35]Aguila LA, Lopes MR, Pretti FZ, et al. Clinical and laboratory features of overlap syndromes of idiopathic inflammatory myopathies associated with systemic lupus erythematosus, systemic sclerosis, or rheumatoid arthritis. Clin Rheumatol. 2014;33(8):1093-1098. [36]Heidari B, Hassanjani Roushan MR. Rheumatoid arthritis and osteoporosis. Caspian J Intern Med. 2012;3(3): 445-446. [37]Chen H, Kubo KY. Bone three-dimensional microstructural features of the common osteoporotic fracture sites. World J Orthop. 2014;18;5(4):486-495. [38]Chen H, Zhou X, Fujita H, et al. Age-related changes in trabecular and cortical bone microstructure. Int J Endocrinol. 2013;2013:213234. [39]Courtland HW, Kennedy OD, Wu Y, et al. Low levels of plasma IGF-1 inhibit intracortical bone remodeling during aging. Age (Dordr). 2013;35(5):1691-1703. [40]Giambini H, Wang HJ, Zhao C, et al. Anterior and posterior variations in mechanical properties of human vertebrae measured by nanoindentation. J Biomech. 2013;46(3): 456-461.[41]Chen H, Zhou X, Washimi Y, et al. Three-dimensional microstructure of the bone in a hamster model of senile osteoporosis. Bone 2008;43:494-500. [42]Popp AW, Buffat H, Eberli U, et al. Microstructural parameters of bone evaluated using HR-pQCT correlate with the DXA-derived cortical index and the trabecular bone score in a cohort of randomly selected premenopausal women. PLoS One. 2014;9(2):e88946. [43]Chiba K, Burghardt AJ, Osaki M, et al. Heterogeneity of bone microstructure in the femoral head in patients with osteoporosis: an ex vivo HR-pQCT study. Bone. 2013;56(1): 139-146.[44]Nicks KM, Amin S, Melton LJ 3rd,et al. Three-dimensional structural analysis of the proximal femur in an age-stratified sample of women. Bone. 2013;55(1):179-188. [45]Manske SL, Liu-Ambrose T, Cooper DM, et al. Cortical and trabecular bone in the femoral neck both contribute to proximal femur failure load prediction. Osteoporos Int. 2009; 20(3):445-453.[46]Hordon LD, Peacock M. The architecture of cancellous and cortical bone in femoral neck fracture. Bone Miner. 1990;11: 335-345. [47]Lang TF, Keyak JH, Heitz MW, et al. Volumetric quantitative computed tomography of the proximal femur: precision and relation to bone strength. Bone. 1997;21:101-108. [48]Riggs BL, Melton LJ 3rd, Robb RA, et al. Population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. J Bone Miner Res. 2004;19:1945-1954. |
| [1] | 黄登承, 王志科, 曹学伟. 体外冲击波疗法治疗中老年膝骨关节炎短期疗效对比的荟萃分析[J]. 中国组织工程研究, 2021, 25(9): 1471-1476. |
| [2] | 韦 玮, 李 剑, 黄林海, 兰敏东, 卢显威, 黄绍东. 全膝或全髋关节置换后老年人首次活动时跌倒恐惧的影响因素[J]. 中国组织工程研究, 2021, 25(9): 1351-1355. |
| [3] | 彭智浩, 冯宗权, 邹勇根, 牛国庆, 吴 峰. 活动平台单髁置换后下肢力线与外侧间室骨关节炎进展的关系[J]. 中国组织工程研究, 2021, 25(9): 1368-1374. |
| [4] | 张 冲, 刘志昂, 姚帅辉, 高军胜, 姜 岩, 张 陆. 局部应用氨甲环酸减少老年股骨颈骨折全髋关节置换后引流的安全和有效性[J]. 中国组织工程研究, 2021, 25(9): 1381-1386. |
| [5] | 杜秀鹏, 杨朝晖. 65岁以下嵌插型股骨颈骨折初始畸形程度对颈缩短的影响[J]. 中国组织工程研究, 2021, 25(9): 1410-1416. |
| [6] | 陈俊名, 岳 辰, 何沛霖, 张俊涛, 孙墨渊, 刘又文. 髋关节置换与股骨近端防旋髓内钉内固定修复高龄股骨转子间骨折效果的Meta分析[J]. 中国组织工程研究, 2021, 25(9): 1452-1457. |
| [7] | 唐 辉, 姚志浩, 罗道文, 彭双麟, 杨双林, 王 浪, 肖金刚. 高脂高糖饮食结合链脲佐菌素建立2型糖尿病性骨质疏松症大鼠模型[J]. 中国组织工程研究, 2021, 25(8): 1207-1211. |
| [8] | 刘翔翔, 黄云梅, 陈文列, 林如辉, 卢小冬, 李钻芳, 许亚晔, 黄美雅, 李西海. 早期骨关节炎模型大鼠半月板白区细胞的超微结构变化[J]. 中国组织工程研究, 2021, 25(8): 1237-1242. |
| [9] | 李中峰, 陈明海, 凡一诺, 魏秋实, 何 伟, 陈镇秋. 右归饮治疗激素性股骨头坏死作用机制的网络药理学分析[J]. 中国组织工程研究, 2021, 25(8): 1256-1263. |
| [10] | 侯广原, 张继学, 张志军, 孟祥晖, 段 文, 高维陆. 骨水泥强化椎弓根螺钉内固定治疗伴骨质疏松腰椎退行性疾病的1年随访[J]. 中国组织工程研究, 2021, 25(6): 878-883. |
| [11] | 李时斌, 赖 渝, 周 毅, 廖建钊, 章晓云, 张 璇. 激素性股骨头坏死发病机制及相关信号通路的靶点效应[J]. 中国组织工程研究, 2021, 25(6): 935-941. |
| [12] | 刘 欣, 颜飞华, 洪坤豪. 调控水通道蛋白表达延缓膝骨关节炎模型大鼠软骨退变[J]. 中国组织工程研究, 2021, 25(5): 668-673. |
| [13] | 马泽涛, 曾 晖, 王德利, 翁 鉴, 冯 松. 微小RNA-138-5p与软骨细胞增殖和自噬的关系[J]. 中国组织工程研究, 2021, 25(5): 674-678. |
| [14] | 曹旭含, 白子兴, 孙承颐, 杨艳军, 孙卫东. “乳香-没药”治疗膝骨关节炎网络药理学分析[J]. 中国组织工程研究, 2021, 25(5): 746-753. |
| [15] | 黎永华, 冯 强, 谭仁霆, 黄世福, 邱金龙, 尹 恒. 杜仲活性成分抗膝骨关节炎滑膜炎病变分子机制的网络药理学阐述[J]. 中国组织工程研究, 2021, 25(5): 765-771. |
中国组织工程研究杂志出版内容重点:组织构建;骨细胞;软骨细胞;细胞培养;成纤维细胞;血管内皮细胞;骨质疏松;组织工程
http://www.crter.org/CN/folder/folder1979.shtml
Design
Histology in vitro and comparative observation.
Time and setting
The experiment was performed at the Orthopaedic Research Laboratory, Department of Orthopaedic Surgery, Odense University Hospital, University of Southern Denmark between September 2011 and September 2014.
Subjects
Human femur neck samples: human proximal femoral necks including heads were harvested from patients undergoing total hip replacements at the Department of Orthopaedic Surgery and Traumatology, Odense University Hospital. These were 7 female patients with rheumatoid arthritis (average age 67.14 years; range 52–86 years), and 10 female patients with osteoarthritis (average age 68.86 years; range 58–82 years). Prior to experiments, all written documents were obtained from patients who agreed to donate femoral necks/heads, and all written informed consents were obtained from all subjects to offer cadavers for anatomical education and research. Thus, this study included two groups: osteoarthritis and rheumatoid arthritis. All patients and donors were Caucasian. The inclusion criteria: a strict criteria for selecting samples was used, all osteoarthritis and rheumatoid arthritis samples had clinical and radiological diagnosis to confirm the diseases. They were also checked with roentgenographic, biochemical or histological evidence to rule out other bone diseases than osteoarthritis and rheumatoid arthritis. Congenital or acquired dysplasia, gout or avascular necrosis were excluded from the osteoarthritis and rheumatoid arthritis groupS. This study was approved by the Department of Orthopaedic Surgery and Traumatology, Odense University Hospital, and the Ethic Committee of the Region of Southern Denmark (ID: S-VF-20040094).
.jpg)
![]()
Methods
Sample preparation
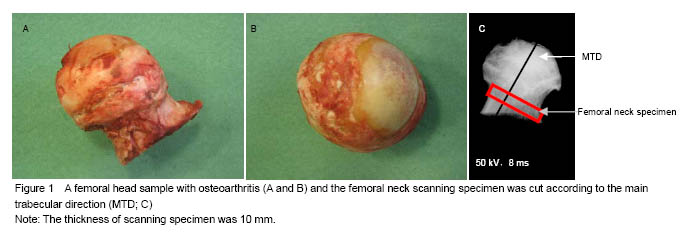
According to total hip replacement procedure, the femur head was sawed off between trachantal minor and major. The femur head samples were stored in sealed plastic bag and kept in -20 ℃ for future preparation. The proximal femurs were cleaned of soft connective tissue with a scalpel. Before the specimens were harvested, the orientation of the trabeculae in the femoral neck was determined from an anterior-posterior contact radiograph. In each X-ray image, the main trabecular direction was identified by the operator and the angle between the tangent line of the inferior cortical bone of the femoral neck and the main trabecular direction was measured. A 10-mm segment of the femoral neck was cut at the base of femoral head and at the base of femoral neck, perpendicular to the main trabecular direction of each individual femur, using Exakt saw (Exakt Apparatebau GmbH & Co. KG, Norderstedt, Germany) (Figure 1).
.jpg)
Micro-CT scanning and three-dimensional microstructural analysis
The specimens were analyzed by cone-beam micro-CT system (vivaCT 40, Scanco Medical AG., Zurich, Switzerland). The entire cortical and cancellous bone was scanned continuously with a tube voltage of 70 kV, tube current of 84 μA. Data from each three-dimensional image consisted of approximately micro-CT slide images (512× 512 pixels) with 16-bit grey levels.
After scanning, the micro-CT image data were transferred to a workstation, and the entire cortical and trabecular region of the femoral head were selected and segmented using an individual density threshold value. The cortical bone was defined by removing the osteophyte at the periosteal surface
operator. We binarized the image to separate pure bone from background or pores, using a single threshold.
of the cortex and the pores at the endosteal surface of the cortex by the same Based on the three-dimensional reconstructions of the total cortical (Co) and trabecular (Tb) bone, mean bone density (MBD, mg HA/ccm), bone surface (BS, mm2), bone volume (BV, mm3), total volume (TV, mm3), thickness (Th*, mm), number (N*, mm-1), separation (Sp,mm), were analyzed. MBD, BS, BV, and TV were calculated using tetrahedrons corresponding to the enclosed volume of the triangulated surface. BS/BV, BS/TV, BV/TV were calculated. Th*, N*, and Sp of the cortical and trabecular bone were based on direct measurement by a distance transformation method. The structure model index (SMI) is a parameter that quantified the characteristic form of a three-dimensional described structure in terms of the plate-like or rod-like nature of the complete structure. Connectivity density (CD, mm-3) is a topological parameter that estimate the number of trabecular connections per cubic millimeter. The degree of anisotropy (DA) defines the direction and magnitude of the preferred orientation of trabeculae and uses the ratio between the maximum and minimum radii of the mean intercept length ellipsoid. All the above parameters were computed in three-dimensional without model assumptions required for two-dimensional analysis. Cross sectional area (CSA) was calculated according to two-dimensional reconstruction data, and the value was the mean value of three different cross sections (top, middle and bottom).
Main outcome measures
The entire microstructure of the femoral neck and measurement indicators.
Statistical analysis
All statistical analyses were completed using SPSS 16.0 software (SPSS, Chicago, IL, USA). Data were presented as mean±SD. Normality of the distributions was assessed using the Kolmogorov-Smirnov test with the significance level set at 0.05. Differences of these properties between the two groups were analyzed using independent-samples t test. The data were not normally distributed, so non-parametric tests (Mann-Whitney U test) were performed. Bivariate correlation analysis and linear regression analysis were conducted to assess the association of different properties between the groups. A value of P < 0.05 was considered significant.
This study investigated the bone microarchitectural changes of the femoral neck in patients with osteoarthritis and rheumatoid arthritis. It was an interesting observation that there were no significant differences in the microarchitectural parameters of the entire femoral neck between osteoarthritis and rheumatoid arthritis groups. These results did not support our hypothesis that the microarchitecture was significantly different between osteoarthritis and rheumatoid arthritis patients. Thus, our data might suggest that osteoarthritis and rheumatoid arthritis had a similar trend of global bone microarchitectural degeneration in the femoral neck, despite marked erosion in rheumatoid arthritis, but the local difference could not be eliminated.
We did not find significant differences in bone quality and trabecular microarchitecture between patients with osteoarthritis and rheumatoid arthritis in integrity, cortical and trabecular bone properties of the femoral neck. To our knowledge, this is the first study evaluating the three-dimensional structure of the femoral neck in patients with rheumatoid arthritis using micro-CT.
It is a surprising finding that the global microarchitecture of the cancellous bone in the femoral neck did not differ between osteoarthritis and rheumatoid arthritis patients, despite apparent erosion in rheumatoid arthritis bone. As it is generally believed that rheumatoid arthritis is a major cause of secondary osteoporosis, and thus the deteriorated microarchitecture of cancellous bone should be typically characterized as decreased bone density (volume fraction), transformation of cancellous bone structure into extremely rod-like. However, our data did not support this assumption. The global changes in cancellous bone and cortical bone were similar in the femoral necks of osteoarthritis and rheumatoid arthritis patients, but this did not propose that local changes were similar as well. Given the fact that rheumatoid arthritis-induced erosion particularly close to the joint surface was apparent, there were significant differences in local microarchitecture of bone tissues. Nevertheless, our current data suggest a similar trend of global microarchitectural degeneration in osteoarthritis and rheumatoid arthritis patients.
Femoral neck fracture is one of the most common fractures in osteoporosis patients. Rheumatoid arthritis is a major cause of secondary osteoporosis and is frequently associated with both paraarticular and generalized osteoporosis[12]. Femoral neck fracture is attributed to the loss of both cortical and trabecular bone mass[30-33]. Bone mass in osteoarthritis patients is higher than that in normal subjects[1]. It is demonstrated that the femoral neck in patients with osteoarthritis has increased cancellous bone area, connectivity and trabecular thickness which may all protect the neck against fractures[9, 29]. Although several studies have found that the microstructural changes of the femoral neck not only exist in the trabecular bone but also in the cortical bone which determine that the bone strength of femoral neck play the crucial role in prevalence of femoral neck fracture, these studies did not investigate the integrity structure of femoral neck[1, 2, 4, 8-10, 20, 29]. This study was the first to measure the three-dimensional microstructural parameters of the entire femoral neck instead of partial specimens from some part of the femoral neck. We think that it is of great significance for assessing the effects of changes in the bone properties on femoral neck fracture.
In this study, the microarchitectural parameters measured were not different between the two groups. The bone volume fraction of the aging control was similar to that of the aging tibial cancellous bone. The structure type of the control group was typical rod-like, and was similar to what was reported earlier in human aging tibial cancellous bone[5]. In general, the decreased bone tissues in the osteoarthritis and rheumatoid arthritis groups were compensated during aging and disease processes, resulting in severe bone loss with aging. According to our data, the entire cortical bone of the femoral neck in patients with rheumatoid arthritis increased by 2.19% in BV/TV, 2.89% in Co.Th*, 2.07% in Co.N* and 26.55% in Co.CD, respectively; declined by 4.16% in Co.Sp, 0.7% in Co.DA compared to osteoarthritis patients. The bone properties of the trabecular bone and the entire femoral neck were similar to those of the cortical bone. The change of BV/TV was associated with change in Co.Th, Co.N, and Co.Sp in our findings which are in line with previous studies[1-2, 5-10].
It seems our findings are not in agreement with the results of previous studies that there are more bone quality in osteoarthritis patients that osteoporosis patients[9, 20, 29]. We think the discrepancy of our findings can be interpreted by several causes. First, in all almost previous studies, for assessing cortical and trabecular bone structures and their possible regional variability in the femoral neck, the osteoporosis specimen was taken from osteoporotic hip fracture (primary osteoporosis) instead of secondary osteoporosis[1-2, 4, 7, 20, 29]. Rheumatoid arthritis is a major cause of secondary osteoporosis and is frequently associated with both paraarticular and generalized osteoporosis. Rheumatoid arthritis is an autoimmune disorder of unknown etiology characterized by progressive damage of synovial-lined joints and variable extra-articular manifestations. Detailed microarchitecture of the cancellous bone and cortical bone in rheumatoid arthritis patients is still not well known[12, 18, 34-35]. The factors of rheumatoid arthritis-induced osteoporosis are more complex than primary osteoporosis. Generalized bone loss may be influenced by immobility, the inflammatory process and treatments such as steroids, while paraarticular loss is probably due to local release of inflammatory agents such as cytokines from the rheumatoid synovium and articular immobility. Determinants of bone mass in rheumatoid arthritis are multifactorial such as sex and menopausal status, disease duration, disease activity, and reduced mobility and function[18-36]. Due to this, it is possibly inapposite that the changes of bone microstructure in rheumatoid arthritis-induced secondary osteoporosis are similar to those in primary osteoporosis. Although there are many studies demonstrating that rheumatoid arthritis shows high bone turnover and the same decreased bone mineral density[12-17], we did not find any studies addressing the difference in three-dimensional bone microstructure between primary osteoporosis and rheumatoid arthritis using micro-CT. Hence, we think if there is another study group of primary osteoporosis as control group, it is very helpful to detect the difference. But it is regretful that it is very difficult to perfectly obtain the entire femoral neck from osteoporotic hip fracture patients due to the operation or fracture damage of the specimen. Secondly, it is well accepted that age-related bone loss is an important factor leading to enhanced bone fragility and fracture risk in the elderly[37-39]. Age-related changes of trabecular bone include a decrease in BV/TV, Tb.N and Conn.D, an increase in Tb.Sp, a shift from plate-like trabeculae to rod-like structure. With normal aging, this thin cortical zone in the femoral neck becomes substantially thinner[40-43]. Although rheumatoid arthritis patients possibly have osteoporotic changes of bone microstructure, patients with rheumatoid arthritis have a mean age of 67.1 years, who are younger than those with primary osteoarthritis (an average age of 68.2 years).
There are several limitations needed to be discussed. Firstly, there was a small sample-size in each group. Apparently, if more samples were included in the study, the results were possibly more convincing. Secondly, no mechanical test was performed, since these valuable samples were used for another study to investigate ultrastructure of bone tissues. The relative contributions of cortical and trabecular bone is important to maintain the bone strength at the femoral neck[23, 31-32]. Hence, our study was the first to scan the entire femoral neck once, and calculated the three-dimensional parameters of whole cortical and trabecular part in every specimen. According to our method not applied in previous studies, we could easily assess the global properties of the femoral neck, cortical bone and trabecular bone, and analyze the difference between osteoarthritis and rheumatoid arthritis. The relative importance of cortical and trabecular bone to the femoral neck strength has been reported in studies that have used several experimental methods[44-47]. All the studies have taken one part of the femoral neck (cortical or trabecular bone) as interesting specimen, which cannot veritably reflect the general change in the femoral neck in human body and produce bias. In human beings, trabecular bone density of the femoral neck declines twice as much as does cortical thickness or cortical bone density[48]. Moreover, an analytical study found that, at the mid-femoral neck, 50% of the applied load, either during gait or during a sideways fall, was supported by the trabecular bone[32]. But in our study there was no difference in the tendency of changes in cortical, trabecular and entire femoral neck both in osteoarthritis and rheumatoid arthritis patients. We think both cortical and trabecular bones play the same important role in the ability of femoral neck to sustain stresses produced by falls. If with mechanical test, we possibly get the association between microarchitectrual properties and mechanical properties in osteoarthritis and rheumatoid arthritis, and know which part of femoral neck play its different role in change of bone strength while with tension or torsion by falls.
In conclusion, this study demonstrated that there were no significant differences in the global microarchitectural parameters of the femoral neck between osteoarthritis and rheumatoid arthritis. These results might suggest that osteoarthritis and rheumatoid arthritis have a similar trend of global microarchitectural degeneration in the femoral neck, despite marked erosion in the bone of rheumatoid arthritis and osteophyte formation in the bone of osteoarthritis, but the local difference cannot be eliminated. The bone loss with aging in the osteoarthritis and rheumatoid arthritis was not as serious as that of osteoporosis according to literature reports, suggesting a compensation effect of the diseases that increase
1 对于骨关节炎和类风湿性关节炎股骨近端骨组织(包括皮质骨与松质骨)的三维微观结构观察的研究国内外均未见报道。 2 文章的目的是对比研究骨性关节炎与类风湿性关节炎间的骨微观结构差异性。 3 实验的创新性在于首次采用高分辨micro-CT技术分析股骨颈部位骨组织的整体、松质骨和皮质骨的微观结构。
中国组织工程研究杂志出版内容重点:组织构建;骨细胞;软骨细胞;细胞培养;成纤维细胞;血管内皮细胞;骨质疏松;组织工程
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||