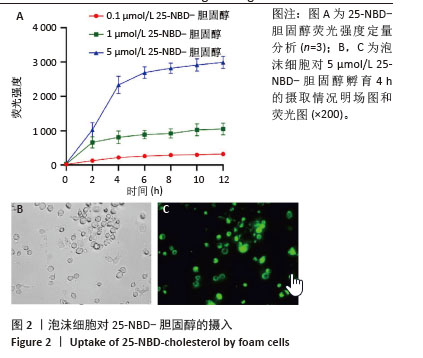
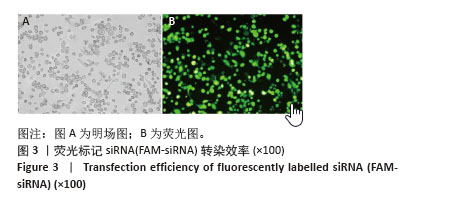
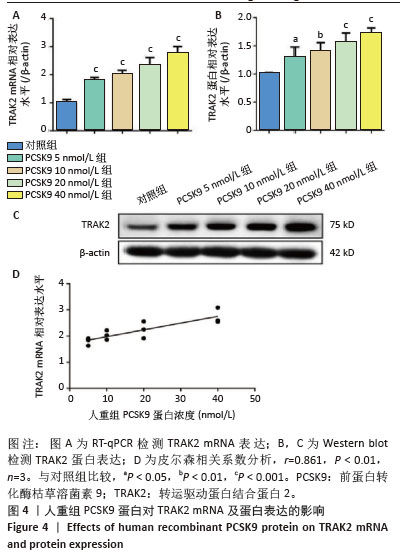
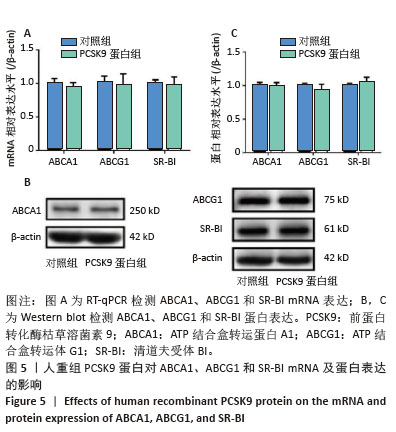
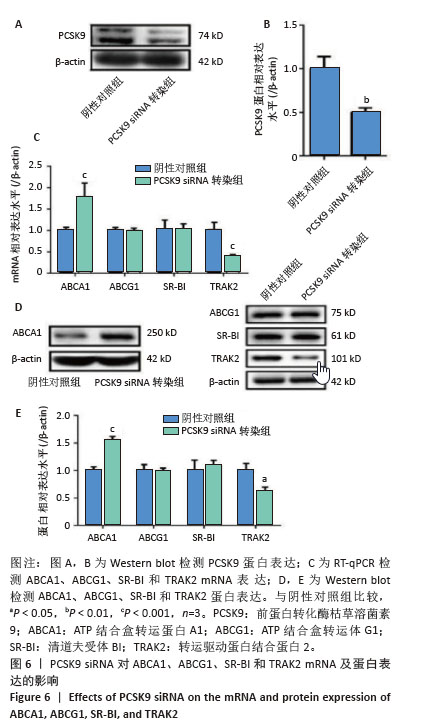
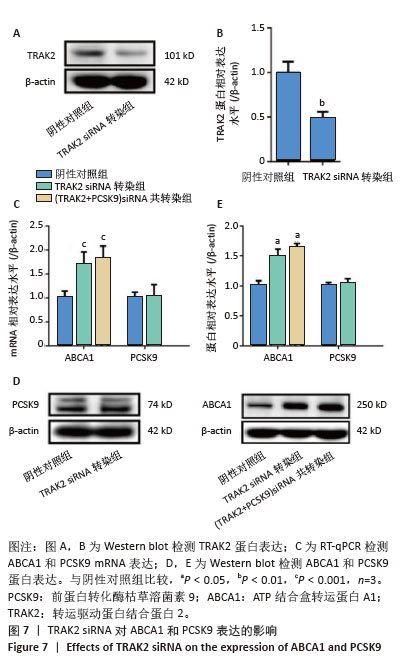
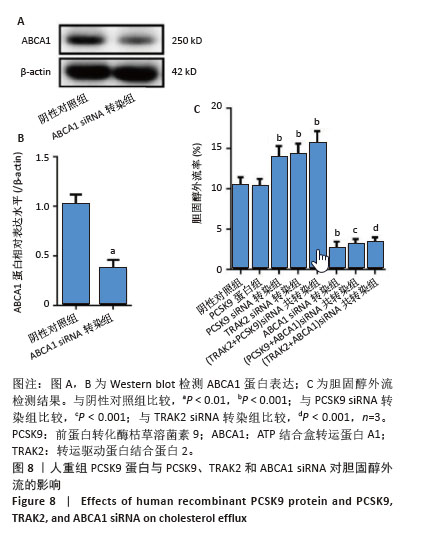
[1] HUMMELGAARD S, VILSTRUP JP, GUSTAFSEN C, et al. Targeting PCSK9 to tackle cardiovascular disease. Pharmacol Ther. 2023; 249:108480.
[2] KATZMANN JL, LAUFS U. PCSK9-directed therapies: an update. Curr Opin Lipidol. 2024;35(3):117-125.
[3] NICHOLLS SJ. PCSK9 inhibitors and reduction in cardiovascular events: Current evidence and future perspectives. Kardiol Pol. 2023;81(2): 115-122.
[4] WANG YM, TAN MY, ZHANG RJ, et al. Acid-Sensing Ion Channel 1/Calpain1 Activation Impedes Macrophage ATP-Binding Cassette Protein A1-Mediated Cholesterol Efflux Induced by Extracellular Acidification. Front Physiol. 2022;12:777386.
[5] ESCOLÀ-GIL JC, ROTLLAN N, JULVE J, et al. Reverse Cholesterol Transport Dysfunction Is a Feature of Familial Hypercholesterolemia. Curr Atheroscler Rep. 2021;23(6):29.
[6] TAO H, YANCEY PG, BLAKEMORE JL, et al. Macrophage SR-BI modulates autophagy via VPS34 complex and PPARα transcription of Tfeb in atherosclerosis. J Clin Invest. 2021;131(7):e94229.
[7] VISHNYAKOVA TG, BOCHAROV AV, BARANOVA IN, et al. SR-BI mediates neutral lipid sorting from LDL to lipid droplets and facilitates their formation. PLoS One. 2020;15(10):e0240659.
[8] LIU X, ZHANG Y, LI X, et al. TRAK2 and Its Interacting Partners in the Regulation of Intracellular Trafficking. PLoS ONE. 2023;18(11): e0277354.
[9] LI X, ZHANG Y, LIU X, et al. The Role of TRAK Proteins in Neuronal Development and Disease. Front Cell Dev Biol. 2021;9:647019.
[10] GANGWAR A, DEODHAR SS, SALDANHA S, et al. Proteomic Determinants of Variation in Cholesterol Efflux: Observations from the Dallas Heart Study. Int J Mol Sci. 2023;24(21):15526.
[11] DE MEYER GRY, ZUREK M, PUYLAERT P, et al. Programmed death of macrophages in atherosclerosis: mechanisms and therapeutic targets. Nat Rev Cardiol. 2024;21(5):312-325.
[12] CAO D, KHAN Z, LI X, et al. Macrophage angiotensin-converting enzyme reduces atherosclerosis by increasing peroxisome proliferator-activated receptor α and fundamentally changing lipid metabolism. Cardiovasc Res. 2023;119(9):1825-1841.
[13] LIBBY P. The changing landscape of atherosclerosis. Nature. 2021; 592(7855):524-533.
[14] MAJDALAWIEH AF, DALIBALTA S, YOUSEF SM. Effects of sesamin on fatty acid and cholesterol metabolism, macrophage cholesterol homeostasis and serum lipid profile: A comprehensive review. Eur J Pharmacol. 2020;885:173417.
[15] YVAN-CHARVET L, IVANOV S. Metabolic Reprogramming of Macrophages in Atherosclerosis: Is It All about Cholesterol? J Lipid Atheroscler. 2020;9(2):231-242.
[16] ADORNI MP, PAPOTTI B, BORGHI MO, et al. Effect of the JAK/STAT Inhibitor Tofacitinib on Macrophage Cholesterol Metabolism. Int J Mol Sci. 2023;24(16):12571.
[17] KONG P, CUI ZY, HUANG XF, et al. Inflammation and atherosclerosis: signaling pathways and therapeutic intervention. Signal Transduct Target Ther. 2022;7(1):131.
[18] KLOC M, KUBIAK JZ, GHOBRIAL RM. Macrophage-, Dendritic-, Smooth Muscle-, Endothelium-, and Stem Cells-Derived Foam Cells in Atherosclerosis. Int J Mol Sci. 2022;23(22):14154.
[19] GUO H, WEI D, LIU R, et al. A novel therapeutic strategy for atherosclerosis: autophagy-dependent cholesterol efflux. J Physiol Biochem. 2022;78(3):557-572.
[20] CHISTIAKOV DA, BOBRYSHEV YV, OREKHOV AN. Macrophage-mediated cholesterol handling in atherosclerosis. J Cell Mol Med. 2016;20(1): 17-28.
[21] 申燕,王沛,周娟,等.慢性肾功能不全患者血清及硫酸吲哚酚对巨噬细胞脂质聚集的影响[J].南方医科大学学报,2015,35(5):631-638.
[22] GUTIERREZ PS. Foam Cells in Atherosclerosis. Arq Bras Cardiol. 2022;119(4):542-543.
[23] ZHOU L, LI C, GAO L, et al. High-density lipoprotein synthesis and metabolism (Review). Mol Med Rep. 2015;12(3):4015-4021.
[24] HARSCH BA, BORKOWSKI K, WALKER RE, et al. ABCA1 and apoA-I dependent 12-hydroxyeicosatetraenoic acid efflux regulates macrophage inflammatory signaling. bioRxiv [Preprint]. 2024: 2024.07.11.603001.
[25] OUIMET M, MARCEL YL. Regulation of lipid droplet cholesterol efflux from macrophage foam cells. Arterioscler Thromb Vasc Biol. 2012;32(3):575-581.
[26] SOBATI S, SHAKOURI A, EDALATI M, et al. PCSK9: A Key Target for the Treatment of Cardiovascular Disease (CVD). Adv Pharm Bull. 2020;10(4):502-511.
[27] MATYAS C, TROJNAR E, ZHAO S, et al. PCSK9, A Promising Novel Target for Age-Related Cardiovascular Dysfunction. JACC Basic Transl Sci. 2023;8(10):1334-1353.
[28] WANG J, XIAO Q, WANG L, et al. Role of ABCA1 in Cardiovascular Disease. J Pers Med. 2022;12(6):1010.
[29] SEIDAH NG, PRAT A. The Multifaceted Biology of PCSK9. Endocr Rev. 2022;43(3):558-582.
[30] LAKE NJ, TAYLOR RL, TRAHAIR H, et al. TRAK2, a novel regulator of ABCA1 expression, cholesterol efflux and HDL biogenesis. Eur Heart J. 2017;38(48):3579-3587.
[31] ADORNI MP, CIPOLLARI E, FAVARI E, et al. Inhibitory effect of PCSK9 on Abca1 protein expression and cholesterol efflux in macrophages. Atherosclerosis. 2017;256:1-6.
[32] ZOU J, XU C, ZHAO ZW, et al. Asprosin inhibits macrophage lipid accumulation and reduces atherosclerotic burden by up-regulating ABCA1 and ABCG1 expression via the p38/Elk-1 pathway. J Transl Med. 2022;20(1):337.
[33] IKONEN E, OLKKONEN VM. Intracellular Cholesterol Trafficking. Cold Spring Harb Perspect Biol. 2023;15(8):a041404.
[34] JIN P, GAO D, CONG G, et al. Role of PCSK9 in Homocysteine-Accelerated Lipid Accumulation in Macrophages and Atherosclerosis in ApoE-/- Mice. Front Cardiovasc Med. 2021;8:746989.
[35] YING Q, RONCA A, CHAN DC, et al. Effect of a PCSK9 inhibitor and a statin on cholesterol efflux capacity: A limitation of current cholesterol-lowering treatments? Eur J Clin Invest. 2022;52(7):e13766.
[36] SONG F, LI JZ, WU Y, et al. Ubiquitinated ligation protein NEDD4L participates in MiR-30a-5p attenuated atherosclerosis by regulating macrophage polarization and lipid metabolism. Mol Ther Nucleic Acids. 2021;26:1303-1317.
[37] FOGELMAN AM, REDDY ST. Making sense of a seemingly odd connection. Eur Heart J. 2017;38(48):3588-3589.
[38] ZHENG S, HUANG H, LI Y, et al. Yin-xing-tong-mai decoction attenuates atherosclerosis via activating PPARγ-LXRα-ABCA1/ABCG1 pathway. Pharmacol Res. 2021;169:105639.
[39] OLADOSU O, ESOBI IC, POWELL RR, et al. Dissecting the Impact of Vascular Smooth Muscle Cell ABCA1 versus ABCG1 Expression on Cholesterol Efflux and Macrophage-like Cell Transdifferentiation: The Role of SR-BI. J Cardiovasc Dev Dis. 2023;10(10):416.
[40] DERGUNOV AD, BASEROVA VB. Different Pathways of Cellular Cholesterol Efflux. Cell Biochem Biophys. 2022;80(3):471-481. |