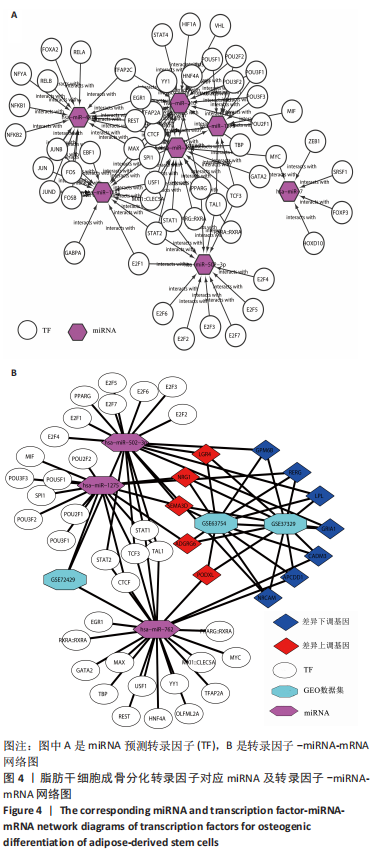
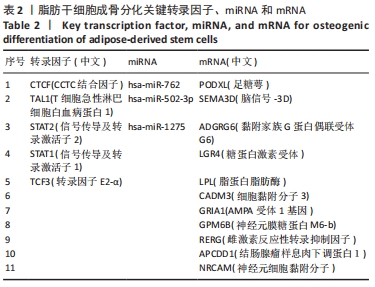
[1] ZHAO X, LIANG M, LI X, et al. Identification of key genes and pathways associated with osteogenic differentiation of adipose stem cells. J Cell Physiol. 2018;233(12):9777-9785.
[2] 李兴艳,杨业静,黄家志,等.脂肪干细胞成骨分化的潜在关键基因与信号通路[J].广西医学,2021,43(1):70-73.
[3] QUAN L, WANG Y, LIANG J, et al. Screening for genes, transcription factors and miRNAs associated with the myogenic and osteogenic differentiation of human adipose tissue-derived stem cells. Int J Mol Med. 2016;38(6):1839-1849.
[4] WARDE-FARLEY D, DONALDSON SL, COMES O, et al. The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. 2010;38(Web Server issue):W214-W220.
[5] HUANG DAW, SHERMAN BT, LEMPICKI RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44-57.
[6] LIU ZP, WU C, MIAO H, et al. RegNetwork: an integrated database of transcriptional and post-transcriptional regulatory networks in human and mouse. Database (Oxford). 2015;2015:bav095.
[7] TOKAR T, PASTRELLO C, ROSSOS AEM, et al. mirDIP 4.1-integrative database of human microRNA target predictions. Nucleic Acids Res. 2018;46(D1):D360-D370.
[8] 陈火. PODXL在胃癌转移中的作用及机制研究[D].苏州:苏州大学,2016.
[9] 胡雪晴,林芙君,吴伟斌,等. Podocalyxin基因沉默对足细胞结构和功能的影响[J].肾脏病与透析肾移植杂志,2018,27(6):538-543.
[10] YUAN WH, XIE QQ, WANG KP, et al. Screening of osteoarthritis diagnostic markers based on immune-related genes and immune infiltration. Sci Rep. 2021;11(1):7032.
[11] RYYN NEN J, KRIEBITZSCH C, MEYER MB, et al. Class 3 semaphorins are transcriptionally regulated by 1,25(OH)(2)D(3) in osteoblasts. J Steroid Biochem Mol Biol. 2017;173:185-193.
[12] SUN P, HE L, JIA K, et al. Regulation of body length and bone mass by Gpr126/Adgrg6. Sci Adv. 2020;6(12):eaaz0368.
[13] YU WJ, ZHANG Z, FU W Z, et al. Association between LGR4 polymorphisms and peak bone mineral density and body composition. J Bone Miner Metab. 2020;38(5):658-669.
[14] LUO J, YANG Z, MA Y, et al. LGR4 is a receptor for RANKL and negatively regulates osteoclast differentiation and bone resorption. Nat Med. 2016;22(5):539-546.
[15] 王斌. Wnt/β-catenin、BMP-2/Runx2/Osterix、LGR4/RANKL/RANK通路的关键因子在骨质疏松性骨折端的表达分析[D].广州:广州医科大学,2019.
[16] CHANG L, GARCIA-ARCOS I, NYR NR, et al. Lipoprotein Lipase Deficiency Impairs Bone Marrow Myelopoiesis and Reduces Circulating Monocyte Levels. Arterioscler Thromb Vasc Biol. 2018;38(3):509-519.
[17] 张晓萌. GPM6B在平滑肌细胞分化中的作用和机制研究[D].西安: 中国人民解放军空军军医大学,2018.
[18] GHARIBI B, GHUMAN MS, CAMA G, et al. Site-specific differences in osteoblast phenotype, mechanical loading response and estrogen receptor-related gene expression. Mol Cell Endocrinol. 2018;477: 140-147.
[19] 史亚茹,杨威利,杨俊英,等. APCDD1对骨髓基质干细胞成脂分化及脂质合成的影响[J].山东医药,2018,58(37):1-5.
[20] RAN S, PEI YF, LIU YJ, et al. Bivariate genome-wide association analyses identified genes with pleiotropic effects for femoral neck bone geometry and age at menarche. PloS One. 2013;8(4):e60362.
[21] 李强.人大肠癌HT-29细胞系Livin基因相关miRNAs的筛选[D].南昌:南昌大学,2014.
[22] 任梦迪,宁玉烨,黎明,等.细粒棘球蚴病患者外周血微小RNA的差异表达分析及其特异性诊断标志物的筛选[J].中国寄生虫学与寄生虫病杂志,2017,35(5):423-428.
[23] GAO J, GAO L, LI R, et al. Integrated analysis of microRNA-mRNA expression in A549 cells infected with influenza A viruses (IAVs) from different host species. Virus Res. 2019;263:34-46.
[24] JOERGER M, BATY F, FR HM, et al. Circulating microRNA profiling in patients with advanced non-squamous NSCLC receiving bevacizumab/erlotinib followed by platinum-based chemotherapy at progression (SAKK 19/05). Lung Cancer. 2014;85(2):306-313.
[25] 王庆辉,钱惠忠,张媛媛,等.基于GEO数据库对慢性乙型肝炎中关键miRNAs筛选及其生物信息学分析[J].山东医药,2020,60(26): 19-23.
[26] RECHSTEINER T, SCHWARZ E, BROCK M, et al. The micro-RNA hsa-miR-502-3p is down-regulated in the plasma of obstructive sleep apnea patients after 2 weeks of continuous positive airway pressure therapy withdrawal: Data from a randomized controlled trial. Eur Respir J. 2014; 44:P2201.
[27] 荆琳,单鹏程,张洪美,等.膝骨关节炎患者软骨组织与血浆中miRNA表达变化及意义[J].山东医药,2016,56(37):61-63.
[28] OZDOGAN H, GUR DEDEOGLU B, OZTEMUR ISLAKOGLU Y, et al. DICER1 gene and miRNA dysregulation in mesenchymal stem cells of patients with myelodysplastic syndrome and acute myeloblastic leukemia. Leuk Res. 2017;63:62-71.
[29] YANG JR, SHI MX, ZENG Y. LncRNA HAND2-AS1 inhibits proliferation and promotes apoptosis of chronic myeloid leukemia cells by sponging with micRNA-1275. Eur Rev Med Pharmacol Sci. 2019;23(5):2103-2111.
[30] MUJAHED H, MILIARA S, NEDDERMEYER A, et al. AML displays increased CTCF occupancy associated with aberrant gene expression and transcription factor binding. Blood. 2020;136(3):339-352.
[31] FAN J, DU W, ZHANG H, et al. Transcriptional downregulation of miR-127-3p by CTCF promotes prostate cancer bone metastasis by targeting PSMB5. FEBS Lett. 2020;594(3):466-476.
[32] PORCHER C, CHAGRAOUI H, KRISTIANSEN M S. SCL/TAL1: a multifaceted regulator from blood development to disease. Blood. 2017;129(15):2051-2060.
[33] DEY S, CURTIS D J, JANE S M, et al. The TAL1/SCL transcription factor regulates cell cycle progression and proliferation in differentiating murine bone marrow monocyte precursors. Mol Cell Biol. 2010;30(9): 2181-2192.
[34] 冯欣,李垚. STAT-1对白介素1β诱导的关节软骨细胞衰老的影响及其可能机制研究[J].中国全科医学,2016,19(11):1310-1313.
[35] YI T, LEE D S, JEON M S, et al. Gene expression profile reveals that STAT2 is involved in the immunosuppressive function of human bone marrow-derived mesenchymal stem cells. Gene. 2012;497(2):131-139.
[36] HEATH H, BRITTON G, KUDO H, et al. Stat2 loss disrupts damage signalling and is protective in acute pancreatitis. J Pathol. 2020;252(1): 41-52.
[37] 谢雪,石毓君,刘玉萍.肝细胞肝癌中TCF3表达及与预后的关系[J].临床与实验病理学杂志,2020,36(9):1036-1042.
[38] HE B, CHEN J, LIU L, et al. Knockdown of Tcf3 enhances the wound healing effect of bone marrow mesenchymal stem cells in rats. Biosci Rep. 2019;39(8):BSR20180369.
[39] GUO Q, LIU Y, SUN R, et al. Mechanical stimulation induced osteogenic differentiation of BMSCs through TWIST/E2A/p21 axis. Biosci Rep. 2020; 40(5):BSR20193876.
|