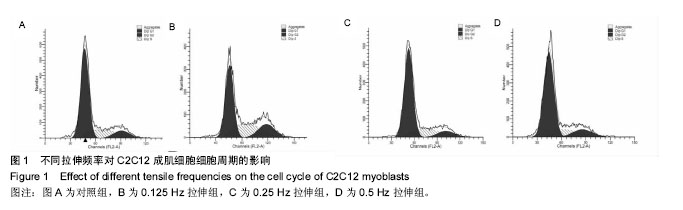
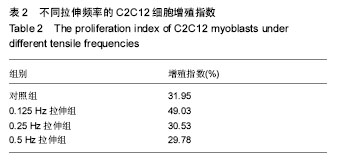
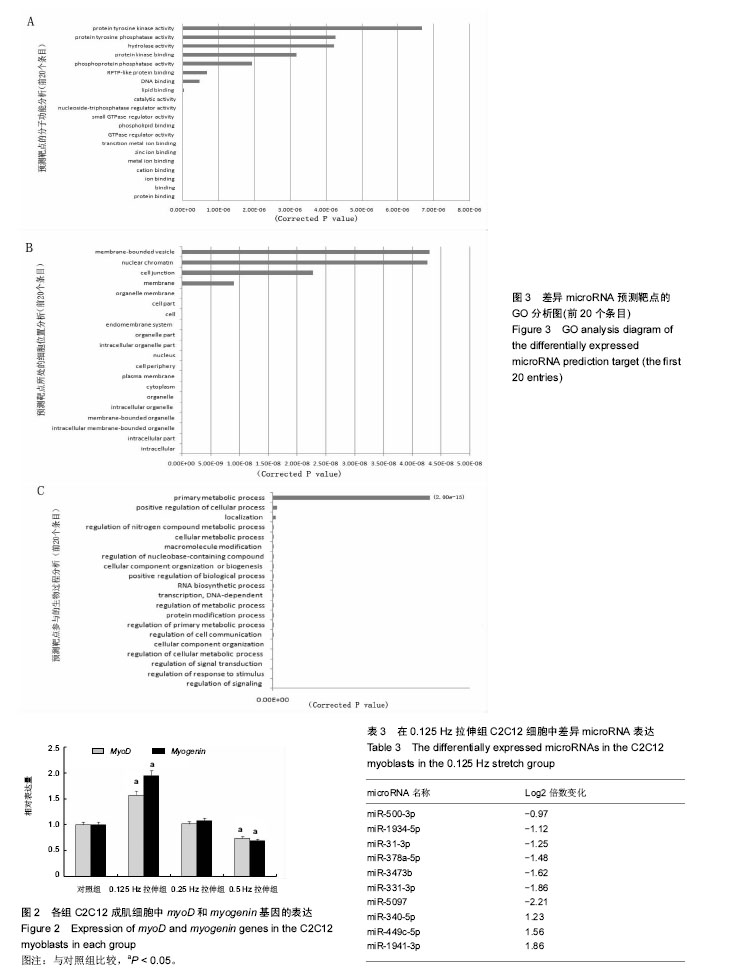
| [1] Kook SH, Lee HJ, Chung WT, et al. Cyclic mechanical stretch stimulates the proliferation of C2C12 myoblasts and inhibits their differentiation via prolonged activation of p38 MAPK. Mol Cells. 2008;25(4): 479-486.[2] Khalsa PS, Ge W, Uddin MZ, et al. Integrin alpha2beta1 affects mechano-transduction in slowly and rapidly adapting cutaneous mechanoreceptors in rat hairy skin. Neuroscience. 2004;129(2): 447-459.[3] Cachaço AS, Pereira CS, Pardal RG, et al. Integrin repertoire on myogenic cells changes during the course of primary myogenesis in the mouse. Dev Dyn. 2005;232(4):1069-1078.[4] Rauch C, Loughna PT. Static stretch promotes MEF2A nuclear translocation and expression of neonatal myosin heavy chain in C2C12 myocytes in a calcineurin- and p38-dependent manner. Am J Physiol Cell Physiol. 2005;288(3): C593-605.[5] Dias P, Dilling M, Houghton P. The molecular basis of skeletal muscle differentiation. Semin Diagn Pathol.1994;11(1): 3-14.[6] Kumar A, Murphy R, Robinson P, et al. Cyclic mechanical strain inhibits skeletal myogenesis through activation of focal adhesion kinase, Rac-1 GTPase, and NF-kappaB transcription factor. FASEB J. 2004;18(13): 1524-1535.[7] Wang H, Sun H, Guttridge DC. microRNAs: novel components in a muscle gene regulatory network. Cell Cycle. 2009;8(12): 1833-1837.[8] Bernstein E, Kim SY, Carmell MA, et al. Dicer is essential for mouse development. Nat Genet. 2003;35(3): 215-217.[9] Li J, Wang G, Jiang J, et al. Dynamical Expression of MicroRNA-127-3p in Proliferating and Differentiating C2C12 Cells. Asian-Australas J Anim Sci. 2016;29(12): 1790-1795.[10] Hua W, Zhang M, Wang Y, et al. Mechanical stretch regulates microRNA expression profile via NF-kappaB activation in C2C12 myoblasts. Mol Med Rep. 2016;14(6): 5084-5092.[11] Ma Z, Sun X, Xu D, et al. MicroRNA, miR-374b, directly targets Myf6 and negatively regulates C2C12 myoblasts differentiation. Biochem Biophys Res Commun. 2015;467(4): 670-675.[12] Zhang Y, Yang L, Gao YF, et al. MicroRNA-106b induces mitochondrial dysfunction and insulin resistance in C2C12 myotubes by targeting mitofusin-2. Mol Cell Endocrinol. 2013; 381(1-2): 230-240.[13] O'Rourke JR, Georges SA, Seay HR, et al. Essential role for Dicer during skeletal muscle development. Dev Biol. 2007; 311(2): 359-368.[14] Wang LY, Wang HY, Ouyang J, et al. Low concentration of lipopolysaccharide acts on MC3T3-E1 osteoblasts and induces proliferation via the COX-2-independent NFkappaB pathway. Cell Biochem Funct, 2009. 27(4): 238-242.[15] Otis JS, Burkholder TJ, Pavlath GK. Stretch-induced myoblast proliferation is dependent on the COX2 pathway. Exp Cell Res. 2005;310(2): 417-425.[16] Wazir R, Luo DY, Dai Y, et al. Expression and proliferation profiles of PKC, JNK and p38MAPK in physiologically stretched human bladder smooth muscle cells. Biochem Biophys Res Commun. 2013;438(3): 479-482.[17] Mok GF, Sweetman D. Many routes to the same destination: lessons from skeletal muscle development. Reproduction. 2011;141(3): 301-312.[18] Yu B, Zhou S, Qian T, et al. Altered microRNA expression following sciatic nerve resection in dorsal root ganglia of rats. Acta Biochim Biophys Sin (Shanghai). 2011;43(11): 909-915.[19] Zhou J, Gao J, Zhang X, et al. microRNA-340-5p Functions Downstream of Cardiotrophin-1 to Regulate Cardiac Eccentric Hypertrophy and Heart Failure via Target Gene Dystrophin. Int Heart J. 2015;56(4): 454-458.[20] Greco S, De Simone M, Colussi C, et al. Common micro-RNA signature in skeletal muscle damage and regeneration induced by Duchenne muscular dystrophy and acute ischemia. FASEB J. 2009;23(10):3335-3346.[21] Cacchiarelli D, Incitti T, Martone J, et al. miR-31 modulates dystrophin expression: new implications for Duchenne muscular dystrophy therapy. EMBO Rep. 2011;12(2):136-141.[22] Crist CG, Montarras D, Buckingham M. Muscle satellite cells are primed for myogenesis but maintain quiescence with sequestration of Myf5 mRNA targeted by microRNA-31 in mRNP granules. Cell Stem Cell. 2012;11(1):118-126.[23] Hou X, Tang Z, Liu H, et al. Discovery of MicroRNAs associated with myogenesis by deep sequencing of serial developmental skeletal muscles in pigs. PLoS One.2012;7(12): e52123.[24] Dmitriev P, Barat A, Polesskaya A, et al. Simultaneous miRNA and mRNA transcriptome profiling of human myoblasts reveals a novel set of myogenic differentiation-associated miRNAs and their target genes. BMC Genomics. 2013;14: 265.[25] Huang Z, Xie X. [Chemerin induces insulin resistance in C2C12 cells through nuclear factor-kappaB pathway-mediated inflammatory reaction]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2015;31(6): 725-729.[26] Zhao M, Zhang ZF, Ding Y, et al. Astragalus polysaccharide improves palmitate-induced insulin resistance by inhibiting PTP1B and NF-kappaB in C2C12 myotubes. Molecules. 2012;17(6):7083-7092.[27] Kemaladewi DU, de Gorter DJ, Aartsma-Rus A, et al. Cell-type specific regulation of myostatin signaling. FASEB J. 2012;26(4): 1462-1472.[28] Xie Q, Deng Y, Huang C, et al. Chemerin-induced mitochondrial dysfunction in skeletal muscle. J Cell Mol Med. 2015;19(5): 986-995.[29] Tabandeh MR, Hosseini SA, Hosseini M, et al. Ginsenoside Rb1 exerts antidiabetic action on C2C12 muscle cells by leptin receptor signaling pathway. J Recept Signal Transduct Res. 2017;37(4): 370-378.[30] Bloch SA, Lee JY, Syburra T, et al. Increased expression of GDF-15 may mediate ICU-acquired weakness by down-regulating muscle microRNAs. Thorax. 2015;70(3): 219-228.[31] Kim SH, Hwang JT, Park HS, et al. Capsaicin stimulates glucose uptake in C2C12 muscle cells via the reactive oxygen species (ROS)/AMPK/p38 MAPK pathway. Biochem Biophys Res Commun. 2013;439(1): 66-70.[32] Tarabees R, Hill D, Rauch C, et al. Endotoxin transiently inhibits protein synthesis through Akt and MAPK mediating pathways in C2C12 myotubes. Am J Physiol Cell Physiol. 2011;301(4): C895-902.[33] Dimchev GA, Al-Shanti N, Stewart CE. Phospho-tyrosine phosphatase inhibitor Bpv(Hopic) enhances C2C12 myoblast migration in vitro. Requirement of PI3K/AKT and MAPK/ERK pathways. J Muscle Res Cell Motil. 2013;34(2): 125-136.[34] Gan L, Liu Z, Zhang Z, et al. SOCS2 inhibited mitochondria biogenesis via inhibiting p38 MAPK/ATF2 pathway in C2C12 cells. Mol Biol Rep. 2014;41(2): 627-637.[35] Zbinden-Foncea H, Deldicque L, Pierre N, et al. TLR2 and TLR4 activation induces p38 MAPK-dependent phosphorylation of S6 kinase 1 in C2C12 myotubes. Cell Biol Int. 2012;36(12): 1107-1113.[36] Lee JO, Kim N, Lee HJ, et al. Visfatin, a novel adipokine, stimulates glucose uptake through the Ca2 +-dependent AMPK-p38 MAPK pathway in C2C12 skeletal muscle cells. J Mol Endocrinol. 2015;54(3): 251-262.[37] 范衡宇, 佟超, 孙青原. 丝裂原活化蛋白激酶(MAPK)信号通路的研究进展[J]. 动物学杂志, 2002, 37(5): 98-102.[38] Knight JD, Tian R, Lee RE, et al. A novel whole-cell lysate kinase assay identifies substrates of the p38 MAPK in differentiating myoblasts. Skelet Muscle. 2012;2: 5.[39] Ji G, Liu D, Liu J, et al. p38 mitogen-activated protein kinase up-regulates NF-kappaB transcriptional activation through RelA phosphorylation during stretch-induced myogenesis. Biochem Biophys Res Commun. 2010;391(1): 547-551.[40] Dimchev GA, Al-Shanti N, Stewart CE. Phospho-tyrosine phosphatase inhibitor Bpv(Hopic) enhances C2C12 myoblast migration in vitro. Requirement of PI3K/AKT and MAPK/ERK pathways. J Muscle Res Cell Motil.2013;34(2):125-136. |
.jpg)

.jpg)
.jpg)