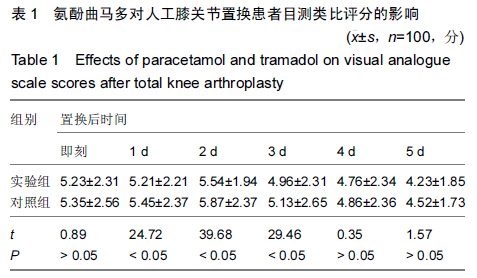
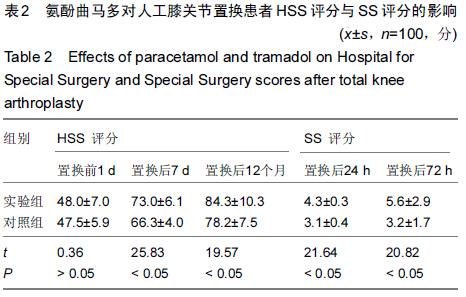
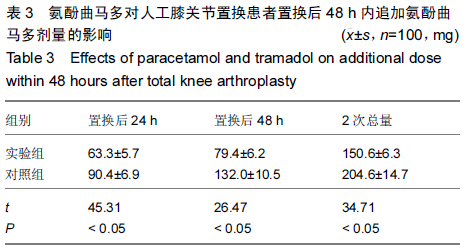
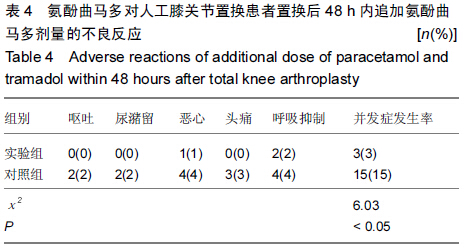
| [1] 尹东,黄宇,莫冰峰,等.环氧化酶-2抑制剂在膝关节置换围手术期镇痛及功能康复的作用[J].中华关节外科杂志:电子版,2014,8(2): 171-174.
[2] 张挺,王睿铸,胡燕军,等.视觉模拟评分法在全膝关节置换术镇痛后应用体会[J].现代生物医学进展,2011,11(22):4362-4363.
[3] 张学政,蒋柳明,徐旭仲,等.帕瑞昔布钠对颌面外科术后芬太尼静脉自控镇痛的影响[J].实用医学杂志,2010,26(20): 3803-3805.
[4] 米坤龙,韦民.人工膝关节置换术后康复与疼痛治疗[J].山西医药杂志,2007,36(4):335-336.
[5] 程凯,李雪萍,于俊龙.全膝关节置换术后的康复治疗要点与相关研究[J].中国康复理论与实践,2006,12(10):875-876.
[6] Lin WQ, Cao LH, Zhong ZJ, et al. Postoperative analgesia with fentanyl combined with flurbiprofen axetil following gynecologic surgery for turnor. Nan Fang Yi Ke Da Xue Xue Bao. 2009; 29(2):313-315.
[7] 朱长江,赵秀梅,刘亚林,等.帕瑞昔布钠超前镇痛对妇科术后自控硬膜外镇痛效果的影响[J].中国误诊学杂志,2011,11(17): 4044-4046.
[8] 叶坪,寇伯龙,张斌.塞来昔布在髋膝关节置换手术后多模式镇痛的应用[J].中华临床医师杂志:电子版,2011,5(3):844-846.
[9] 陈焕诗,金伟,许峰.人工全膝关节置换术治疗膝关节骨关节炎[J].实用骨科杂志,2009,15(7):540-541.
[10] 凌晓芳,倪穗琴.氨酚曲马多片治疗骨科术后疼痛的疗效观察[J].临床医学工程,2010,17(8):85-86.
[11] 江海燕,袁巍.早期大角度被动ROM练习对全膝置换术后膝关节功能的影响[J].中国康复医学杂志,2008,23(10):924-927.
[12] 金勋杰,李贵涛,陈文坚,等.氨酚曲马多治疗骨科中重度疼痛的疗效及安全性的研究[J].中华关节外科杂志,2009,3(3):68-89.
[13] 陈珣,张剑锋,王彩燕,等.氨酚曲马多片剂用于治疗妇科手术后疼痛的临床观察[J].临床麻醉学杂志,2008,24(1):42-43.
[14] Wegener JT, van Ooij B, van Dijk CN, et al. Value of single-injection or continuous sciatic nerve block in addition to a continuous femoral nerve block in patients undergoing total knee arthroplasty: a prospective, randomized, controlled trial. Reg Anesth Pain Med. 2011;36(5):481-488.
[15] Bertin P, Keddad K, Jolivet-Landreau I. Acetaminophen as symptomatic treatment of pain from osteoarthritis. Joint Bone Spine. 2004;71(4):266-274.
[16] 肖宏,林世和,杨述华,等.氨酚曲马多片治疗骨科门急诊中、重度疼痛1683例[J].中国新药与临床杂志,2010,29(12):908-912.
[17] 杨晓燕,田晓娟,刘泽源.复方氨酚曲马多片中对乙酰氨基酚的人体药代动力学研究[J].国际药学研究杂志,2009,36(4):287-295.
[18] Ostapowicz G, Fontana RJ, Schiødt FV, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137(12): 947-954.
[19] Wang SY, Olson-Kellogg B, Shamliyan TA, et al. Physical therapy interventions for knee pain secondary to osteoarthritis: a systematic review. Ann Intern Med. 2012;157(9):632-644.
[20] 吴林飞,徐旭仲,董小秋,等.超前镇痛对全膝关节置换术后疼痛及患者血浆P物质和血清IL-6的影响[J].苏州大学学报(医学版), 2010,30(3):608-610.
[21] 张俊,蒋垚,邵俊杰,等.人工全膝关节置换术中关节周围注射镇痛药物和术后持续性冷冻压迫的疗效分析[J].中国骨与关节损伤杂志,2008,23(3):205-207.
[22] 鞠洪斌,余存泰,覃健,等.全膝关节置换术中关节周围局部注药镇痛效果的研究[J].中国矫形外科杂志,2007,15(13):967-968.
[23] Horlocker TT, Kopp SL, Pagnano MW, et al. Analgesia for total hip and knee arthroplasty: a multimodal pathway featuring peripheral nerve block. J Am Acad Orthop Surg. 2006;14(3):126-135.
[24] 曾金才,孙俊英,杨立文,等.关节内置管局部浸润镇痛在全膝关节置换术的应用[J].中国矫形外科杂志,2009,17(21):1609-1611.
[25] 高正玉,王英振,徐红梅,等.膝关节局部注药在全膝关节置换镇痛中的应用[J].中国矫形外科杂志,2009,17(9):654-656.
[26] 文俊,刘君,邓泽彦,等.氨酚羟考酮片对全膝置换术后患者镇痛效果的临床观察[J].山西医药杂志,2010,39(1):78-80.
[27] Ittichaikulthol W, Prachanpanich N, Kositchaiwat C, et al. The post-operative analgesic efficacy of celecoxib compared with placebo and parecoxib after total hip or knee arthroplasty. J Med Assoc Thai. 2010;93(8):937-942.
[28] 张县华,张洪,周一新,等.全膝关节置换术围手术期多模式镇痛方案的临床研究[J].中华骨科杂志,2008,28(8):647-650.
[29] 符培亮,吴字黎,吴海山,等.全膝置换术后关节内注射鸡尾酒式镇痛混合剂对镇痛效果的评价[J].中华骨科杂志,2008,28(7): 541-545.
[30] 高升焘,孙爱娟,张鹏,等.全膝关节置换术后应用连续股神经阻滞镇痛和静脉自控镇痛的效果比较[J].中华关节外科杂志:电子版, 2012,6(1):54-58.
[31] 陆捷,张晓丽,倪雪珺.超前镇痛对全膝关节置换术后患者镇痛效果的影响[J].上海医学,2011,34(2):114-117.
[32] 高升焘,孙爱娟,张鹏,等.全膝关节置换术后应用连续股神经阻滞镇痛和静脉自控镇痛的效果比较[J].中华关节外科杂志(电子版), 2012,6(1):36-39.
[33] 秦维龙,孟庆才,方锐,等.中医药镇痛方案在人工膝关节置换术围手术期的疗效观察[J].中医药导报,2010,16(10):43-44.
[34] 刘静,解雪,张其亮,等.疼痛控制对人工全膝关节置换术后患者早期康复效果的影响[J].中华护理杂志,2010,45(6):512-514.
[35] Edwards JE, McQuay HJ, Moore RA. Combination analgesic efficacy: individual patient data meta-analysis of single-dose oral tramadol plus acetaminophen in acute postoperative pain. J Pain Symptom Manage. 2002;23(2):121-130.
[36] 刘川.氨酚曲马多片治疗髂胫束综合征的临床价值[J].中国现代医药杂志,2012,3(7):112.
[37] 管大伟,李艳,王超,等.氨酚曲马多在人工全膝关节围置换期镇痛中的作用及安全性[J].中国组织工程研究,2012,16(26): 4781-4785.
[38] 黄少芬.曲马多缓释片治疗骨折术后疼痛的效果观察[J].中国当代医药,2010,31(27):231.
[39] 丁悦,曾展鹏,许杰,等.氨酚曲马多治疗骨科急慢性疼痛的疗效[J].中国实用医药,2010,5(25):123.
[40] 荀乙峰,王燕飞.氨酚曲马多治疗带状疱疹及带状疱疹后遗神经痛疗效观察[J].中国实用医药,2011,6(7):131.
[41] 黄诚.氨酚曲马多治疗骨科腰椎术后疼痛观察[J].中国医药指南, 2012,10(10):196.
[42] Dahl JB, Mathiesen O, Møiniche S. 'Protective premedication': an option with gabapentin and related drugs? A review of gabapentin and pregabalin in in the treatment of post-operative pain. Acta Anaesthesiol Scand. 2004;48(9):1130-1136.
[43] Dirks J, Fredensborg BB, Christensen D, et al. A randomized study of the effects of single-dose gabapentin versus placebo on postoperative pain and morphine consumption after mastectomy. Anesthesiology. 2002;97(3):560-564.
[44] Pandey CK, Priye S, Singh S, et al. Preemptive use of gabapentin significantly decreases postoperative pain and rescue analgesic requirements in laparoscopic cholecystectomy. Can J Anaesth. 2004;51(4):358-363.
[45] Pandey CK, Singhal V, Kumar M, et al. Gabapentin provides effective postoperative analgesia whether administered pre-emptively or post-incision. Can J Anaesth. 2005;52(8): 827-831.
[46] 谢冰柯,许乐.疼痛控制影响因素的研究进展[J].国际护理学杂志,2007,26(7):677-680.
[47] 支秀玲,李艳红,梁文丽.疼痛的护理评估及控制进展[J].护理研究,2003,17(2A):139-140.
[48] 陈新谦,金有豫,汤光.新编药物学[M].15版.北京:人民卫生出版社, 2003:2212-2222.
[49] 刘金荣,于丽.术后患者静脉自控镇痛的临床护理[J].中国当代医学,2007,15:110.
[50] Powell AE, Davies HT, Bannister J, et al. Rhetoric and reality on acute pain services in the UK: a national postal questionnaire survey. Br J Anaesth. 2004;92(5):689-693.
[51] 孙贵豫,许吟,李兰,等.应用自控镇痛泵的护理[J].护士进修杂志, 2004,19(5):476.
[52] 张啟维,薛庆云,王强,等.帕瑞昔布在后路腰椎融合术后多模式镇痛中的临床应用[J].中国脊柱脊髓杂志,2010,20(10):855-859.
[53] Sommer M, de Rijke JM, van Kleef M, et al. The prevalence of postoperative pain in a sample of 1490 surgical inpatients. Eur J Anaesthesiol. 2008;25(4):267-274.
[54] 王锦琰.超前镇痛面临的挑战[J].中国疼痛医学杂志,2005,11(6): 321-322.
[55] 赵继军,崔静.护士在疼痛管理中的作用[J].中华护理杂志,2009, 44(4):383. |