中国组织工程研究 ›› 2019, Vol. 23 ›› Issue (27): 4402-4407.doi: 10.3969/j.issn.2095-4344.1393
• 组织构建临床实践 clinical practice in tissue construction • 上一篇 下一篇
痰热清注射液联合盐酸氨溴索治疗老年退行性心脏病心力衰竭致肺部感染:随机,平行,对照,临床试验研究
赵光强
- (三亚市人民医院呼吸内科,海南省三亚市 572000)
Tanreqing injection combined with ambroxol hydrochloride in treating heart failure and pulmonary infection caused by senile degenerative heart disease: a parallel, randomized, controlled clinical trial
Zhao Guangqiang
- (Department of Respiratory Medicine, Sanya People’s Hospital, Sanya 572000, Hainan Province, China)
摘要:
文章快速阅读:
.jpg)
文题释义:
盐酸氨溴索:可促进呼吸道内部黏稠分泌物的排除及减少黏液的滞留,具有促进排痰的功效,临床上常用于治疗呼吸道感染。
痰热清注射液:中国中医复方痰热清注射液的主要成分由黄芩、熊胆粉、金银花、山羊角和连翘组成,具有清热、化痰和解毒的药理活性。
.jpg)
文题释义:
盐酸氨溴索:可促进呼吸道内部黏稠分泌物的排除及减少黏液的滞留,具有促进排痰的功效,临床上常用于治疗呼吸道感染。
痰热清注射液:中国中医复方痰热清注射液的主要成分由黄芩、熊胆粉、金银花、山羊角和连翘组成,具有清热、化痰和解毒的药理活性。
摘要
背景:老年心脏病退行性变化和机体免疫功能的降低,使老年心力衰竭患者容易受到病原菌的感染。中国传统复方中药痰热清注射液具有清热、化痰和解毒的作用,可用于治疗肺部感染。
目的:试验假设采用痰热清注射液联合黏痰溶解药物盐酸氨溴索治疗老年退行性心脏病致心力衰竭伴肺部感染患者,可有效减轻肺部感染,提高心功能。
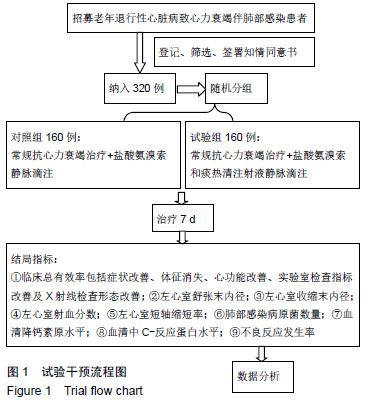
方法:方案设计为前瞻性、单中心、随机、平行、对照、临床研究。纳入三亚市人民医院老年退行性心脏病心力衰竭致肺部感染患者320例,采用随机数字表法分为2组,均进行常规抗心力衰竭治疗,对照组160例(50%)加用盐酸氨溴索静脉滴注,试验组160例(50%)加用盐酸氨溴索和痰热清注射液静脉滴注,共治疗7 d。试验对象招募和资料收集时间为2019-07-01/2021-08-30,结果分析时间为2021-09-10/30,试验完成时间为2021-12-30。试验经中国海南省三亚市人民医院医学伦理委员会批准,批准号:S2015-065-03号,批准时间:2015年10月。研究符合世界医学会制定的《赫尔辛基宣言》的要求;参与者家属或本人均对试验方案和过程知情同意,并签署知情同意书。
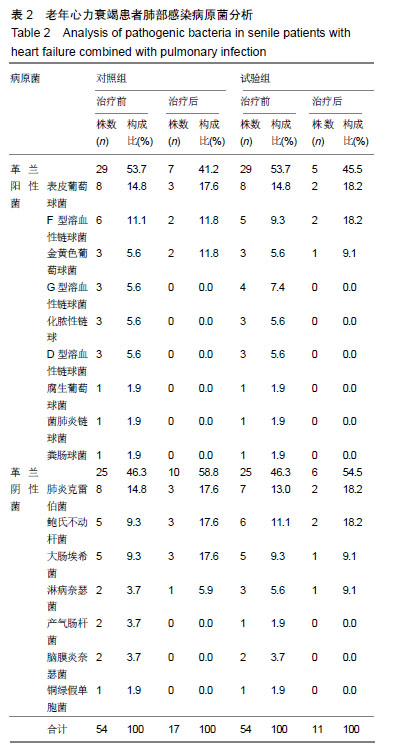
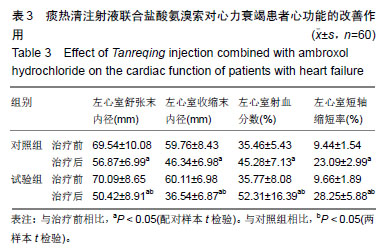
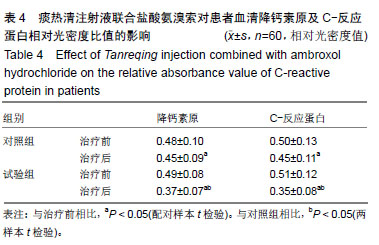
结果与结论:①研究的主要结局指标为治疗后7 d临床总有效率;②次要结局指标为治疗前、治疗后7 d左心室收缩末内径、左心室舒张末内径、左心室射血分数、左心室短轴缩短率、肺部感染病原菌数量、血清中降钙素原及C-反应蛋白水平变化;治疗后7 d不良反应发生率;③课题组于前期(2016年5月至2017年10月)已完成了老年心力衰竭患者120例小样本试验,随机分为试验组和对照组各60例,均占50%,结果发现,老年心力衰竭患者肺部感染病原菌以革兰阳性菌为主,对照组和试验组均为53.7%,治疗后试验组患者的病原菌数目明显低于对照组患者病原菌的数目(对照组:试验组=17株:11株);与治疗前相比,试验组和对照组患者治疗后左心室收缩末内径,左心室舒张末内径及左心室射血分数均明显降低(P < 0.05),而左心室短轴缩短率水平明显升高(P < 0.05),血清降钙素原及C-反应蛋白水平均明显降低(P < 0.05),且试验组上述指标变化更显著(P < 0.05);④试验希望证实,痰热清注射液联合盐酸氨溴索可明显改善老年心力衰竭患者的心功能,并能明显减轻病原菌致肺部感染,在此明确由于心脏病退行性改变而发生的特征性药物不良反应。试验已在中国临床试验注册中心注册(注册号:ChiCTR1900022879),注册时间:2019-04-29,方案版本号1.0,注册成功后开始纳入患者,研究成果科在国内外相关会议报告,可在国内外相关开放获取杂志发表。
背景:老年心脏病退行性变化和机体免疫功能的降低,使老年心力衰竭患者容易受到病原菌的感染。中国传统复方中药痰热清注射液具有清热、化痰和解毒的作用,可用于治疗肺部感染。
目的:试验假设采用痰热清注射液联合黏痰溶解药物盐酸氨溴索治疗老年退行性心脏病致心力衰竭伴肺部感染患者,可有效减轻肺部感染,提高心功能。
方法:方案设计为前瞻性、单中心、随机、平行、对照、临床研究。纳入三亚市人民医院老年退行性心脏病心力衰竭致肺部感染患者320例,采用随机数字表法分为2组,均进行常规抗心力衰竭治疗,对照组160例(50%)加用盐酸氨溴索静脉滴注,试验组160例(50%)加用盐酸氨溴索和痰热清注射液静脉滴注,共治疗7 d。试验对象招募和资料收集时间为2019-07-01/2021-08-30,结果分析时间为2021-09-10/30,试验完成时间为2021-12-30。试验经中国海南省三亚市人民医院医学伦理委员会批准,批准号:S2015-065-03号,批准时间:2015年10月。研究符合世界医学会制定的《赫尔辛基宣言》的要求;参与者家属或本人均对试验方案和过程知情同意,并签署知情同意书。
结果与结论:①研究的主要结局指标为治疗后7 d临床总有效率;②次要结局指标为治疗前、治疗后7 d左心室收缩末内径、左心室舒张末内径、左心室射血分数、左心室短轴缩短率、肺部感染病原菌数量、血清中降钙素原及C-反应蛋白水平变化;治疗后7 d不良反应发生率;③课题组于前期(2016年5月至2017年10月)已完成了老年心力衰竭患者120例小样本试验,随机分为试验组和对照组各60例,均占50%,结果发现,老年心力衰竭患者肺部感染病原菌以革兰阳性菌为主,对照组和试验组均为53.7%,治疗后试验组患者的病原菌数目明显低于对照组患者病原菌的数目(对照组:试验组=17株:11株);与治疗前相比,试验组和对照组患者治疗后左心室收缩末内径,左心室舒张末内径及左心室射血分数均明显降低(P < 0.05),而左心室短轴缩短率水平明显升高(P < 0.05),血清降钙素原及C-反应蛋白水平均明显降低(P < 0.05),且试验组上述指标变化更显著(P < 0.05);④试验希望证实,痰热清注射液联合盐酸氨溴索可明显改善老年心力衰竭患者的心功能,并能明显减轻病原菌致肺部感染,在此明确由于心脏病退行性改变而发生的特征性药物不良反应。试验已在中国临床试验注册中心注册(注册号:ChiCTR1900022879),注册时间:2019-04-29,方案版本号1.0,注册成功后开始纳入患者,研究成果科在国内外相关会议报告,可在国内外相关开放获取杂志发表。
中图分类号:
.jpg)
.jpg) #br#
文题释义:#br#
盐酸氨溴索:可促进呼吸道内部黏稠分泌物的排除及减少黏液的滞留,具有促进排痰的功效,临床上常用于治疗呼吸道感染。#br#
痰热清注射液:中国中医复方痰热清注射液的主要成分由黄芩、熊胆粉、金银花、山羊角和连翘组成,具有清热、化痰和解毒的药理活性。
#br#
文题释义:#br#
盐酸氨溴索:可促进呼吸道内部黏稠分泌物的排除及减少黏液的滞留,具有促进排痰的功效,临床上常用于治疗呼吸道感染。#br#
痰热清注射液:中国中医复方痰热清注射液的主要成分由黄芩、熊胆粉、金银花、山羊角和连翘组成,具有清热、化痰和解毒的药理活性。