| [1]René R.Postmenopausal Osteoporosis: assessment and management. Best Pract Res Clin Endocrinol Metab. 2018; 32(5):739-757.[2]及金宝, 郑淑萍.绝经后骨质疏松症的治疗进展[J]. 世界临床药物, 2018,39(9):647-650.[3]Michael LE. Treatment of osteoporosis with denosumab. Maturitas.2010;66(2):182-186.[4]Coskun Benlidayi I. Denosumab in the treatment of glucocorticoid-induced osteoporosis. Rheumatol Int. 2018; 38(11):1975-1984.[5]Altay MA, Radu AD, Pack SE, et al.Medication-related osteonecrosis of the jaw: An institution's experience.Cranio. 2018;9:1-9.[6]Diz P, López-Cedrún JL, Arenaz J, et al.Denosumab-related osteonecrosis of the jaw. JADA.2012;143(9):981-984.[7]Kostenuik PJ,Nguyen HQ, McCabe J,et al. Denosumab, a fully human monoclonal antibody to RANKL, inhibits bone resorption and increases BMD in knock-in mice that express chimeric (murine/human) RANKL.J Bone Miner Res.2009; 24(2):182-195.[8]Fontalis A, Kenanidis E, Prousali E, et al. Safety and efcacy of denosumab in osteoporotic patients previously treated with other medications: a systematic review and meta-analysis. Expert Opin Drug Saf. 2018,17(4):413-428.[9]Lewiecki EM.New and emerging concepts in the use of denosumab for the treatment of osteoporosis. Ther Adv Musculoskelet Dis. 2018;10(11):209-223..[10]Cao X.RANKL-RANK signaling regulates osteoblast differentiation and bone formation.Bone Res.2018;6(35): 426-427.[11]Chitre M,Shechter D,Grauer A.Denosumab for treatment of postmenopausal osteoporosis.Am J Health Syst Pharm. 2011;68(15):1409-1418.[12]Pittman K, Antill Y C, Goldrick A, et al. Denosumab: Prevention and management of hypocalcemia, osteonecrosis of the jaw and atypical fractures. Asia Pac J Clin Oncol.2017; 13(4):266-276.[13]Otto S, Pautke C, Van den Wyngaert T, et al.Medication- related osteonecrosis of the jaw: Prevention, diagnosis and management in patients with cancer and bone metastases. Cancer Treat Rev. 2018;69:177-187.[14]Khan AA,Morrison A,Hanley DA,et al.Diagnosis and Management of Osteonecrosis of the Jaw: A Systematic Review and International Consensus. J Bone Miner Res. 2015;30(1):3-23. [15]王杞章. 药物性颌骨坏死的研究进展[J].华西口腔医学杂志, 2018,36(5):568-572.[16]许伟建. 药物相关性颌骨骨坏死:文献回顾与6例病例报告[D]. 杭州:浙江大学, 2017.[17]Ruggiero SL,Dodson TB,Fantasia J.American Association of Oral and Maxillofacial Surgeons Position Paper on Medication-Related Osteonecrosis of the Jaw—2014 Update. J Oral Maxillofac Surg. 2014,72(10):1938-1956.[18]Wat WZM. Current Controversies on the Pathogenesis of Medication-Related Osteonecrosis of the Jaw.Dent J (Basel). 2016 Oct 28;4(4).[19]Anastasilakis AD,Polyzos SA,Makras P.THERAPY OF ENDOCRINE DISEASE: Denosumab vs bisphosphonates for the treatment of postmenopausal osteoporosis. Eur J Endocrinol. 2018 ;179(1):R31-R45. [20]Yang H,Pan H,Yu F,et al.A novel model of bisphosphonate- related osteonecrosis of the jaw in rats.Int J Clin Exp Pathol. 2015;8(5):5161-5167.[21]Saad F, Brown J E, Van Poznak C, et al.Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Ann Oncol.2012;23(5): 1341-1347.[22]Reid IR, Miller PD, Brown JP. Effects of denosumab on bone histomorphometry: the FREEDOM and STAND studies.J Bone Miner Res.2010;25(10):2256-2265.[23]Fizazi K,Carducci M,Smith M,et al.Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet.2011;377(9768):813-822.[24]谢冰洁,冯捷,韩向龙.破骨细胞生物学特征的研究与进展[J].中国组织工程研究, 2017,21(11):1770-1775.[25]Brown JP,Prince RL,Deal C,et al.Comparison of the effect of denosumab and alendronate on BMD and biochemical markers of bone turnover in postmenopausal women with low bone mass: a randomized, blinded, phase 3 trial.J Bone Miner Res.2009;24(1):153-161.[26]Higuchi T,Soga Y,Muro M,et al.Replacing zoledronic acid with denosumab is a risk factor for developing osteonecrosis of the jaw. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;125(6): 547-551.[27]Rahim I, Salt S, Heliotis M.Successful long-term mandibular reconstruction and rehabilitation using non-vascularised autologous bone graft and recombinant human BMP-7 with subsequent endosseous implant in a patient with bisphosphonate-related osteonecrosis of the jaw. Br J Oral Maxillofac Surg. 2015;53(9):870-874.[28]Stresing V, Fournier P G, Bellahcene A, et al. Nitrogen-containing bisphosphonates can inhibit angiogenesis in vivo without the involvement of farnesyl pyrophosphate synthase.Bone. 2011;48(2):259-266.[29]Pabst AM,Ziebart T,Ackermann M.Bisphos-phonates' ?antiangiogenic?potency?in?the?development?of? bisphosphonate-associated? osteonecrosis? of? the?jaws:?influence?on?microvessel?sprouting?in?an in vivo 3D? Matrigel? assay.Clin? Oral? Invest.2014;18(3):1015-1022.[30]李彦博.双膦酸盐和下颌骨坏死[J]. 现代肿瘤医学, 2012,20(5): 1065-1069.[31]Misso G,Porru M,Stoppacciaro A,et al.Evaluation of the in vitro and in vivo antiangiogenic effects of denosumab and zoledronic acid.Cancer Biol Ther.2012;13(14):1491-1500.[32]Leibbrandt A,Penninger JM.TNF conference 2009: beyond bones - RANKL/RANK in the immune system. Adv Exp Med Biol.2011;691:5-22.[33]Bishop KA,Coy HM,Nerenz RD.Mouse Rankl expression is regulated in T cells by c-Fos through a cluster of distal regulatory enhancers designated the T cell control region. J Biol Chem. 2011 Jun 10;286(23):20880-20891.[34]Watts NB,Roux C, Modlin J F, et al.Infections in postmenopausal women with osteoporosis treated with denosumab or placebo : Coincidence or causal association. Osteoporos Int.2012;23(1):327-337.[35]Soutome S, Hayashida S, Funahara M, et al. Factors affecting development of medication-related osteonecrosis of the jaw in cancer patients receiving high-dose bisphosphonate or denosumab therapy: Is tooth extraction a risk factor?.PLOS ONE.2018;13(7):e201343.[36]Boquete-Castro A,Gomez-Moreno G,Calvo-Guirado JL,et al.Denosumab and osteonecrosis of the jaw. A systematic analysis of events reported in clinical trials.Clin Oral Implants Res. 2016,27(3):367-375.[37]车路阳,刘长振,黄鹏.RANKL/RANK/OPG通路及其相关药物狄诺塞麦治疗骨质疏松的研究进展[J].世界最新医学信息文摘, 2017,17(34):43-46.[38]Ohga N, Sato J, Asaka T, et al.Successful conservative treatment of jaw osteonecrosis caused by denosumab in patients with multiple bone metastasis. J Oral Sci. 2018;60(1): 159-162.[39]Pichardo SE, van Merkesteyn JP. Evaluation of a surgical treatment of denosumab-related osteonecrosis of the jaws. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122(3):272-278.[40]Hoefert S,Yuan A,Munz A,et al.Clinical course and therapeutic outcomes of operatively and non-operatively managed patients with denosumab-related osteonecrosis of the jaw (DRONJ). J Craniomaxillofac Surg. 2017;45(4):570-578. [41]Voss PJ,Steybe D,Poxleitner P,et al.Osteonecrosis of the jaw in patients transitioning from bisphosphonates to denosumab treatment for osteoporosis.Odontology.2018;106(4):469-480.[42]苏程.药物相关性颌骨坏死的研究进展[J]. 国际口腔医学杂志, 2017,44(2):228-234.[43]Spanou A,Lyritis GP, Chronopoulos E, et al. Management of bisphosphonate-related osteonecrosis of the jaw: a literature review. Oral Diseases.2015;21(8):927-936.[44]郭玉兴,郭传瑸.二膦酸盐相关颌骨骨坏死临床治疗研究进展[J].中国实用口腔科杂志, 2016,9(3):178-181.[45]Emma D.Deeks. Denosumab: A Review in Postmenopausal Osteoporosis. Drugs Aging. 2018;35(2):163-173.[46]Tsourdi E,Zillikens MC.Certainties and Uncertainties About Denosumab Discontinuation. Calcif Tissue Int.2018;103(1):1-4.[47]McClung MR,Wagman RB,Miller PD,et al.Observations following discontinuation of long-term denosumab therapy. Osteoporos Int.2017;28(5):1723-1732.[48]Zanchetta MB, Boailchuk J, Massari F,et al.Significant bone loss after stopping long-term denosumab treatment: a post FREEDOM study.Osteoporos Int.2018;29(1):41-47.[49]Anastasilakis AD,Polyzos SA,Makras P,et al.Clinical Features of 24 Patients With Rebound-Associated Vertebral Fractures After Denosumab Discontinuation: Systematic Review and Additional Cases.J Bone Miner Res.2017;32(6):1291-1296.[50]Maryam I,Crystal D,Pilar M,et al.Effects of Denosumab After Treatment Discontinuation : A Review of the Literature. Consult Pharm. 2018;33(3):142-151. [51]Katsarelis H,Shah NP,Dhariwal DK,et al.Infection and medication-related osteonecrosis of the jaw.J Dent Res.2015; 94(4):534-539.[52]Owosho AA,Blanchard A,Levi L, et al. Osteonecrosis of the jaw in patients treated with denosumab for metastatic tumors to the bone: A series of thirteen patients.J Craniomaxillofac Surg. 2016;44(3):265-270.[53]Matsumoto A,Sasaki M,Schmelzeisen R,et al.Primary wound closure after tooth extraction for prevention of medication- related osteonecrosis of the jaw in patients under denosumab. Clin Oral Investig.2017;21(1):127-134.[54]Chan BH,Yee R,Puvanendran R, et al. Medication-related osteonecrosis of the jaw in osteoporotic patients: prevention and management. Singapore Med J. 2018;59(2):70-75. |
.jpg) 文题释义:
颌骨坏死:①颌面部区域有暴露的骨骼,时间持续8周以上;②有抗吸收剂治疗史;③颅面部没有接受过放射治疗。
骨免疫学:骨细胞和免疫细胞都起源于骨髓,两者共享多种细胞因子及受体,并通过复杂的相互作用共同调节骨代谢的平衡,骨骼系统及免疫系统之间密切的病理生理联系构成了骨免疫学的主要内容。
文题释义:
颌骨坏死:①颌面部区域有暴露的骨骼,时间持续8周以上;②有抗吸收剂治疗史;③颅面部没有接受过放射治疗。
骨免疫学:骨细胞和免疫细胞都起源于骨髓,两者共享多种细胞因子及受体,并通过复杂的相互作用共同调节骨代谢的平衡,骨骼系统及免疫系统之间密切的病理生理联系构成了骨免疫学的主要内容。.jpg) 文题释义:
颌骨坏死:①颌面部区域有暴露的骨骼,时间持续8周以上;②有抗吸收剂治疗史;③颅面部没有接受过放射治疗。
骨免疫学:骨细胞和免疫细胞都起源于骨髓,两者共享多种细胞因子及受体,并通过复杂的相互作用共同调节骨代谢的平衡,骨骼系统及免疫系统之间密切的病理生理联系构成了骨免疫学的主要内容。
文题释义:
颌骨坏死:①颌面部区域有暴露的骨骼,时间持续8周以上;②有抗吸收剂治疗史;③颅面部没有接受过放射治疗。
骨免疫学:骨细胞和免疫细胞都起源于骨髓,两者共享多种细胞因子及受体,并通过复杂的相互作用共同调节骨代谢的平衡,骨骼系统及免疫系统之间密切的病理生理联系构成了骨免疫学的主要内容。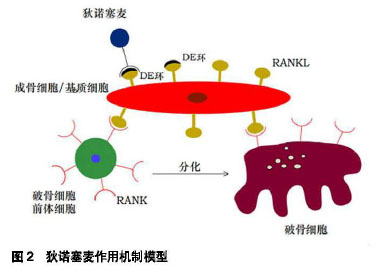

.jpg)
.jpg) 文题释义:
颌骨坏死:①颌面部区域有暴露的骨骼,时间持续8周以上;②有抗吸收剂治疗史;③颅面部没有接受过放射治疗。
骨免疫学:骨细胞和免疫细胞都起源于骨髓,两者共享多种细胞因子及受体,并通过复杂的相互作用共同调节骨代谢的平衡,骨骼系统及免疫系统之间密切的病理生理联系构成了骨免疫学的主要内容。
文题释义:
颌骨坏死:①颌面部区域有暴露的骨骼,时间持续8周以上;②有抗吸收剂治疗史;③颅面部没有接受过放射治疗。
骨免疫学:骨细胞和免疫细胞都起源于骨髓,两者共享多种细胞因子及受体,并通过复杂的相互作用共同调节骨代谢的平衡,骨骼系统及免疫系统之间密切的病理生理联系构成了骨免疫学的主要内容。