中国组织工程研究 ›› 2019, Vol. 23 ›› Issue (23): 3734-3738.doi: 10.3969/j.issn.2095-4344.1321
• 组织构建临床实践 clinical practice in tissue construction • 上一篇 下一篇
实验室相关指标预测肝衰竭患者临床结局的可行性
陈 军,汤 净,陈卜钰
- (海南医学院第一附属医院消化内科,海南省海口市 570000)
Feasibility of laboratory-related indicators in predicting clinical outcome of liver failure
Chen Jun, Tang Jing, Chen Buyu
- (Department of Gastroenterology, the First Affiliated Hospital of Hainan Medical University, Haikou 570000, Hainan Province, China)
摘要:
文章快速阅读:
.jpg) 文题释义:
国际标准化比值(international normalized ratio,INR):是患者凝血酶原时间与正常对照凝血酶原时间之比的ISI次方(ISI:国际敏感度指数,试剂出厂时由厂家标定),是可以校正凝血活酶试剂差异对凝血酶原时间测值进行标准化报告的方法。同一份标本在不同的实验室,用不同的ISI试剂检测,血浆凝血酶原时间值结果差异很大,但测的INR值相同,这样使测得结果具有可比性。目前国际上强调用INR监测口服抗凝药的用量,是一种较好的表达方式。
终末期肝病模型:自2000年美国Malinchoc等最初创立终末期肝病模型(Model for end-stage liver disease,MELD)评分以来,随后的研究证实其为不同的终末期肝病生存率的预测指标。其可对终末期肝病短期、中期死亡率进行有效的预测,评分越高提示患者的预后越差。
文题释义:
国际标准化比值(international normalized ratio,INR):是患者凝血酶原时间与正常对照凝血酶原时间之比的ISI次方(ISI:国际敏感度指数,试剂出厂时由厂家标定),是可以校正凝血活酶试剂差异对凝血酶原时间测值进行标准化报告的方法。同一份标本在不同的实验室,用不同的ISI试剂检测,血浆凝血酶原时间值结果差异很大,但测的INR值相同,这样使测得结果具有可比性。目前国际上强调用INR监测口服抗凝药的用量,是一种较好的表达方式。
终末期肝病模型:自2000年美国Malinchoc等最初创立终末期肝病模型(Model for end-stage liver disease,MELD)评分以来,随后的研究证实其为不同的终末期肝病生存率的预测指标。其可对终末期肝病短期、中期死亡率进行有效的预测,评分越高提示患者的预后越差。
.jpg) 文题释义:
国际标准化比值(international normalized ratio,INR):是患者凝血酶原时间与正常对照凝血酶原时间之比的ISI次方(ISI:国际敏感度指数,试剂出厂时由厂家标定),是可以校正凝血活酶试剂差异对凝血酶原时间测值进行标准化报告的方法。同一份标本在不同的实验室,用不同的ISI试剂检测,血浆凝血酶原时间值结果差异很大,但测的INR值相同,这样使测得结果具有可比性。目前国际上强调用INR监测口服抗凝药的用量,是一种较好的表达方式。
终末期肝病模型:自2000年美国Malinchoc等最初创立终末期肝病模型(Model for end-stage liver disease,MELD)评分以来,随后的研究证实其为不同的终末期肝病生存率的预测指标。其可对终末期肝病短期、中期死亡率进行有效的预测,评分越高提示患者的预后越差。
文题释义:
国际标准化比值(international normalized ratio,INR):是患者凝血酶原时间与正常对照凝血酶原时间之比的ISI次方(ISI:国际敏感度指数,试剂出厂时由厂家标定),是可以校正凝血活酶试剂差异对凝血酶原时间测值进行标准化报告的方法。同一份标本在不同的实验室,用不同的ISI试剂检测,血浆凝血酶原时间值结果差异很大,但测的INR值相同,这样使测得结果具有可比性。目前国际上强调用INR监测口服抗凝药的用量,是一种较好的表达方式。
终末期肝病模型:自2000年美国Malinchoc等最初创立终末期肝病模型(Model for end-stage liver disease,MELD)评分以来,随后的研究证实其为不同的终末期肝病生存率的预测指标。其可对终末期肝病短期、中期死亡率进行有效的预测,评分越高提示患者的预后越差。摘要
背景:终末期肝病模型因受主观因素影响较小等原因在近年来被广泛应用,但有研究表明其在肝衰竭患者预后的评估中优势不明显。
目的:分析实验室相关指标在肝衰竭临床结局预测中的应用价值。
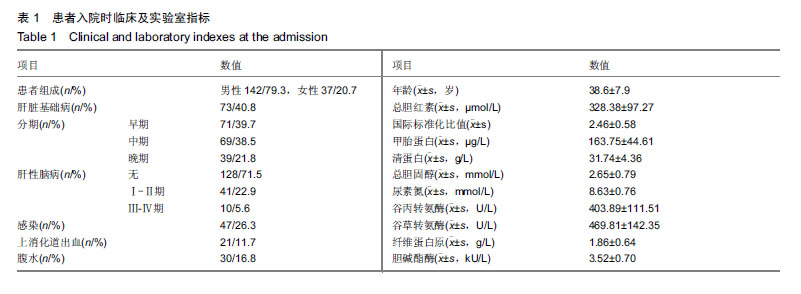
方法:选取海南医学院第一附属医院消化内科收治肝衰竭患者179例,男142例,女37例;平均年龄(38.6±7.9)岁。研究方案及试验设计经海南医学院第一附属医院伦理委员会批准,参与试验的患病个体及其家属均签署知情同意书。根据肝衰竭分期患者分为早、中、晚期;按患者入院8周预后分为生存组和死亡组。收集患者实验室相关指标,通过Logistic多因素回归分析筛选影响肝衰竭临床结局的危险因素,分析各指标与患者分期的关系,并使用ROC曲线分析危险因素对肝衰竭临床结局的预测能力。
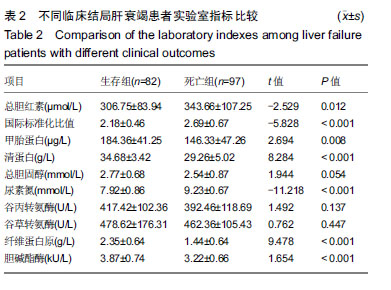
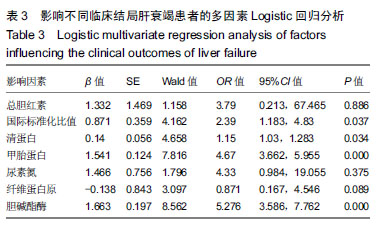
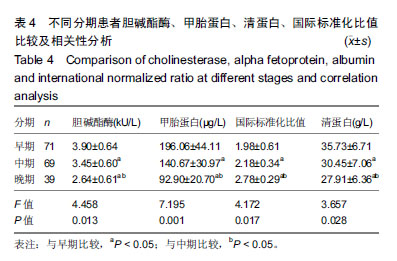
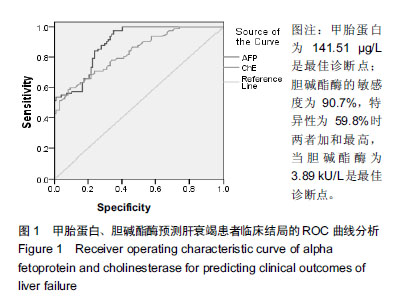
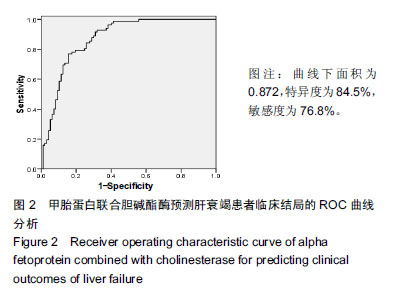
结果与结论:①不同临床结局肝衰竭患者实验室指标比较:生存组总胆红素、国际标准化比值和尿素氮均显著低于死亡组;而甲胎蛋白、清蛋白、纤维蛋白原和胆碱酯酶均高于死亡组(均P < 0.05);②Logistic回归分析结果显示:国际标准化比值、清蛋白、甲胎蛋白和胆碱酯酶对肝衰竭临床结局存在影响;根据OR值,对肝衰竭临床结局的影响最大前2项是胆碱酯酶和甲胎蛋白;③不同分期实验室指标比较:早、中、晚3期患者胆碱酯酶、甲胎蛋白和清蛋白血清学指标呈下降趋势,国际标准化比值呈上升趋势(P < 0.05),不同分期患者胆碱酯酶、甲胎蛋白、国际标准化比值和清蛋白水平两两比较,差异有显著性意义(P < 0.05);不同病情分期与胆碱酯酶、甲胎蛋白、清蛋白负相关(均P < 0.001);国际标准化比值与患者病情分期呈正相关(r=0.548,P < 0.001);④ROC曲线分析:甲胎蛋白的最佳诊断点为141.51 μg/L,其敏感度为97.6%,特异性为64.9%;胆碱酯酶最佳诊断点为3.89 kU/L,敏感度为90.7%,特异性为59.8%;甲胎蛋白联合胆碱酯酶预测肝衰临床结局,曲线下面积为0.872,特异度为84.5%,敏感度为76.8%;⑤结果说明,胆碱酯酶及甲胎蛋白可用于预测肝衰竭患者临床结局,二者联合可以对肝衰竭临床结局作出较为准确的预测。
中图分类号:
.jpg) 文题释义:
国际标准化比值(international normalized ratio,INR):是患者凝血酶原时间与正常对照凝血酶原时间之比的ISI次方(ISI:国际敏感度指数,试剂出厂时由厂家标定),是可以校正凝血活酶试剂差异对凝血酶原时间测值进行标准化报告的方法。同一份标本在不同的实验室,用不同的ISI试剂检测,血浆凝血酶原时间值结果差异很大,但测的INR值相同,这样使测得结果具有可比性。目前国际上强调用INR监测口服抗凝药的用量,是一种较好的表达方式。
终末期肝病模型:自2000年美国Malinchoc等最初创立终末期肝病模型(Model for end-stage liver disease,MELD)评分以来,随后的研究证实其为不同的终末期肝病生存率的预测指标。其可对终末期肝病短期、中期死亡率进行有效的预测,评分越高提示患者的预后越差。
文题释义:
国际标准化比值(international normalized ratio,INR):是患者凝血酶原时间与正常对照凝血酶原时间之比的ISI次方(ISI:国际敏感度指数,试剂出厂时由厂家标定),是可以校正凝血活酶试剂差异对凝血酶原时间测值进行标准化报告的方法。同一份标本在不同的实验室,用不同的ISI试剂检测,血浆凝血酶原时间值结果差异很大,但测的INR值相同,这样使测得结果具有可比性。目前国际上强调用INR监测口服抗凝药的用量,是一种较好的表达方式。
终末期肝病模型:自2000年美国Malinchoc等最初创立终末期肝病模型(Model for end-stage liver disease,MELD)评分以来,随后的研究证实其为不同的终末期肝病生存率的预测指标。其可对终末期肝病短期、中期死亡率进行有效的预测,评分越高提示患者的预后越差。