| [1] Smalley KS,Herlyn M.Towards the targeted therapy of melanoma.Mini Rev Med Chem.2006;6(4):387-393.[2] Langer R.Drug delivery and targeting.Nature.1998;392(6679 Suppl): 5-10.[3] Gowda R,Jones NR,Banerjee S,et al.Use of Nanotechnology to Develop Multi-Drug Inhibitors For Cancer Therapy.J Nanomed Nanotechnol.2013;4(6).pii:184.[4] Wang J,Lee JS,Kim D,et al.Exploration of Zinc Oxide Nanoparticles as a Multi-target and Multi-functional Anticancer Nanomedicine.ACS Appl Mater Interfaces. 2017;9(46):39971-39984.[5] Liu J,Kang Y,Yin S,et al.Zinc oxide nanoparticles induce toxic responses in human neuroblastoma SHSY5Y cells in a size-dependent manner.Int J Nanomedicine. 2017;12:8085-8099.[6] Sasidharan A,Chandran P,Menon D,et al.apid dissolution of ZnO nanocrystals in acidic cancer microenvironment leading to preferential apoptosis.Nanoscale.2011;3(9):3657-3669.[7] Sirelkhatim A,Mahmud S,Seen A,et al.Preferential cytotoxicity of ZnO nanoparticle towards cervical cancer cells induced by ROS-mediated apoptosis and cell cycle arrest for cancer therapy.J Nanopart Res. 2016;18(8):219.[8] Zhang Y,Nayak TR,Hong H,et al.Biomedical applications of zinc oxide nanomaterials.Curr Mol Med. 2013;13(10):1633-1645.[9] Zhang AP,Sun YP.Photocatalytic killing effect of TiO2 nanoparticles on Ls-174-t human colon carcinoma cells.World J Gastroenterol. 2004; 10(21):3191-3193.[10] Wason MS,Colon J,Das S,et al.Sensitization of pancreatic cancer cells to radiation by cerium oxide nanoparticle-induced ROS production. Nanomedicine.2013;9(4):558-569.[11] Shen C,James SA,de Jonge MD,et al.Relating cytotoxicity, zinc ions, and reactive oxygen in ZnO nanoparticle-exposed human immune cells.Toxicol Sci.2013;136(1:120-130.[12] Narayanan KB,Sakthive Nl.Biological synthesis of metal nanoparticles by microbes.Adv Colloid Interface Sci.2010;156(1-2):1-13.[13] Bhattacharyya S,Kudgus RA,Bhattacharya R,et al.Inorganic nanoparticles in cancer therapy, Pharm Res. 2011;28(2):237-259.[14] Ba DP, Zhang XF,Zhang GL,et al.Zinc oxide nanoparticles induce apoptosis and autophagy in human ovarian cancer cells.Int J Nanomedicine.2017;12:6521-6535.[15] Arakha M,Roy J,Nayak PS,et al.Zinc oxide nanoparticle energy band gap reduction triggers the oxidative stress resulting into autophagy- mediated apoptotic cell death.Free Radic Biol Med. 2017;110:42-53.[16] Roy R,Singh SK,Chauhan LK,et al.Zinc oxide nanoparticles induce apoptosis by enhancement of autophagy via PI3K/Akt/mTOR inhibition. Toxicol Lett.2014;227(1):29-40.[17] Yu KN,Yoon TJ,Minai-Tehrani A,et al.Zinc oxide nanoparticle induced autophagic cell death and mitochondrial damage via reactive oxygen species generation.Toxicol In Vitro. 2013;27(4):1187-1195.[18] Xia T,Kovochich M,Liong M,et al.Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties.ACS Nano. 2008;2(10): 2121-2134.[19] Ezhuthupurakkal PB,Ariraman S,Arumugam S,et al.Anticancer potential of ZnO nanoparticle-ferulic acid conjugate on Huh-7 and HepG2 cells and diethyl nitrosamine induced hepatocellular cancer on Wistar albino rat.Nanomedicine.2018;14(2):415-428. [20] Hassan HF,Mansour AM,Abo-Yousse AMf,et al.Zinc oxide nanoparticles as a novel anticancer approach; in vitro and in vivo evidence.Clin Exp Pharmacol Physiol. 2017;44(2):235-243.[21] Chakraborti S,Chakraborty S,Saha S,et al.PEG-functionalized zinc oxide nanoparticles induce apoptosis in breast cancer cells through reactive oxygen species-dependent impairment of DNA damage repair enzyme NEIL2.Free Radic Biol Med.2017;103:35-47.[22] Boroumand Moghaddam A, Moniri M,Azizi S,et al.Eco-Friendly Formulated Zinc Oxide Nanoparticles: Induction of Cell Cycle Arrest and Apoptosis in the MCF-7 Cancer Cell Line.Genes (Basel). 2017;8(10). pii: E281.doi:10.3390/genes8100281.[23] Paino IM,J Gonçalves F,Souza FL,et al.Zinc Oxide Flower-Like Nanostructures That Exhibit Enhanced Toxicology Effects in Cancer Cells.ACS Appl Mater Interfaces.2016;8(48):32699-32705.[24] Moon SH,Choi WJ,Choi SW,et al.Anti-cancer activity of ZnO chips by sustained zinc ion release.Toxicol Rep. 2016;3:430-438. [25] Al-Ajmi MF,Hussain A,Ahmed F.Novel synthesis of ZnO nanoparticles and their enhanced anticancer activity: Role of ZnO as a drug carrier. Ceramic Int.2016;42(3):4462-4469.[26] Wahab R,Siddiqui MA,Saquib Q,et al.ZnO nanoparticles induced oxidative stress and apoptosis in HepG2 and MCF-7 cancer cells and their antibacterial activity.Colloids Surf B Biointerfaces. 2014;117: 267-276. [27] Setyawati MI,Tay CY,Leong DT.Effect of zinc oxide nanomaterials- induced oxidative stress on the p53 pathway. Biomaterials. 2013; 34(38):10133-10142. [28] Daniel AG,Peterson EJ,Farrell NP.The bioinorganic chemistry of apoptosis: potential inhibitory zinc binding sites in caspase-3.Angew Chem Int Ed Engl.2014;53(16):4098-4101.[29] Kao YY,Chen YC,Cheng TJ,et al.Zinc oxide nanoparticles interfere with zinc ion homeostasis to cause cytotoxicity.Toxicol Sci. 2012; 125(2):462-472.[30] Wahab R,Kaushik NK,Verma AK,et al.Fabrication and growth mechanism of ZnO nanostructures and their cytotoxic effect on human brain tumor U87, cervical cancer HeLa, and normal HEK cells.J Biol Inorg Chem.2011;16(3):431-442.[31] Ng KW,Khoo SP, Heng BC,et al.The role of the tumor suppressor p53 pathway in the cellular DNA damage response to zinc oxide nanoparticles. Biomaterials.2011;32(32):8218-8225.[32] Ahamed M,Akhtar MJ,Raja M,et al.ZnO nanorod-induced apoptosis in human alveolar adenocarcinoma cells via p53, survivin and bax/bcl-2 pathways: role of oxidative stress. Nanomedicine. 2011;7(6):904-913.[33] Hackenberg S,Scherzed A,Kessler M,et al.Zinc oxide nanoparticles induce photocatalytic cell death in human head and neck squamous cell carcinoma cell lines in vitro.Int J Oncol.2010;37(6):1583-1590.[34] Chandrasekaran M,Pandurangan M.In Vitro Selective Anti-Proliferative Effect of Zinc Oxide Nanoparticles Against Co-Cultured C2C12 Myoblastoma Cancer and 3T3-L1 Normal Cells.Biol Trace Elem Res.2016;172(1):148-154.[35] Akhtar MJ,Ahamed M,Kumar S,et al.Zinc oxide nanoparticles selectively induce apoptosis in human cancer cells through reactive oxygen species.Int J Nanomedicine.2012;7:845-857.[36] Ostrovsky S,Kazimirsky G,Gedanken A,et al.Selective cytotoxic effect of ZnO nanoparticles on glioma cells.Nano Res.2009;2(11):882-890.[37] Hanley C,Layne J,Punnoose A,et al.Preferential killing of cancer cells and activated human T cells using ZnO nanoparticles. Nanotechnology. 2008;19(29):295103.[38] Brandt EG,Hellgren M,Brinck T,et al.Molecular dynamics study of zinc binding to cysteines in a peptide mimic of the alcohol dehydrogenase structural zinc site.Phys Chem Chem Phys.2009;11(6):975-983.[39] Rasmussen JW,Martinez E,Louka P,et al.Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications.Expert Opin Drug Deliv.2010;7(9):1063-1077.[40] Ho E.Zinc deficiency, DNA damage and cancer risk.J Nutr Biochem. 2004;15(10):572-578.[41] Cho Y,Gorina S,Jeffrey PD,et al.Crystal structure of a p53 tumor suppressor-DNA complex: understanding tumorigenic mutations. Science.1994;265(5170):346-355.[42] Dhawan DK,Chadha VD.Zinc: a promising agent in dietary chemoprevention of cancer.Indian J Med Res. 2010;132:676-682.[43] Kagara N,Tanaka N,Noguchi S,et al.Zinc and its transporter ZIP10 are involved in invasive behavior of breast cancer cells.Cancer Sci.2007; 98(5):692-697.[44] Bisht G,Rayamajhi S.ZnO Nanoparticles: A Promising Anticancer Agent. Nanobiomedicine.2016;3:9.[45] Maret W.Zinc in Cellular Regulation: The Nature and Significance of "Zinc Signals".Int J Mol Sci. 2017;18(11).pii: E2285.doi: 10.3390/ijms18112285.[46] Ho E,Ames BN.Low intracellular zinc induces oxidative DNA damage, disrupts p53, NFkappa B, and AP1 DNA binding, and affects DNA repair in a rat glioma cell line.Proc Natl Acad Sci U S A. 2002;99(26): 16770-16775.[47] Zowczak M,Iskra M,Torlinski L,et al.Analysis of serum copper and zinc concentrations in cancer patients.Biol Trace Elem Res. 2001;82(1-3): 1-8.[48] Abnet CC,Lai B,Qiao YL,et al.Zinc concentration in esophageal biopsy specimens measured by x-ray fluorescence and esophageal cancer risk.J Natl Cancer Inst.2005;97(4):301-306.[49] Costello LC,Franklin RB,Feng P,et al.Zinc and prostate cancer: a critical scientific, medical, and public interest issue (United States). Cancer Causes Control.2005;16(8):901-915.[50] Turney TW,Duriska MB,Jayaratne V,et al.Formation of zinc-containing nanoparticles from Zn(2)(+) ions in cell culture media: implications for the nanotoxicology of ZnO.Chem Res Toxicol.2012;25(10):2057-2066.[51] Chasapis CT,Loutsidou AC,Spiliopoulou CA,et al.Zinc and human health: an update, Arch Toxicol.2012;86(4):521-534.[52] Casey JR,Grinstein S,Orlowski J.Sensors and regulators of intracellular pH.Nat Rev Mol Cell Biol.2010;11(1):50-61.[53] Manke A,Wang L,Rojanasakul Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity, Biomed Res Int.2013;2013:942916.[54] Wilson MR,Lightbody JH,Donaldso K,et al.Interactions between ultrafine particles and transition metals in vivo and in vitro.Toxicol Appl Pharmacol.2002;184(3):172-179.[55] Song W,Zhang J,Guo J,et al.Role of the dissolved zinc ion and reactive oxygen species in cytotoxicity of ZnO nanoparticles.Toxicol Lett.2010;199(3):389-397.[56] Zhang J,Qin X,Wang B,et al. Zinc oxide nanoparticles harness autophagy to induce cell death in lung epithelial cells.Cell Death Dis. 2017;8(7):e2954.[57] Kawanishi S,Hiraku Y,Murata M,et al.The role of metals in site-specific DNA damage with reference to carcinogenesis, Free Radic Biol Med. 2002;32(9):822-832.[58] Shi H,Hudson LG,Liu KJ.Oxidative stress and apoptosis in metal ion-induced carcinogenesis.Free Radic Biol Med. 2004;37(5):582-593.[59] Valavanidis A,Vlachogianni T,Fiotakis C.8-hydroxy-2' -deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis.J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27(2): 120-139.[60] Heim J,Felder E,Tahir MN,et al.Genotoxic effects of zinc oxide nanoparticles.Nanoscale.2015;7(19):8931-8938.[61] Johnson BM,Fraietta JA,Gracias DT,et al.Acute exposure to ZnO nanoparticles induces autophagic immune cell death. Nanotoxicology. 2015;9(6):737-748.[62] Davis ME,Chen ZG,Shin DM.Nanoparticle therapeutics: an emerging treatment modality for cancer.Nat Rev Drug Discov.2008;7(9):771-782.[63] Abercrombie M,Ambrose EJ.The surface properties of cancer cells: a review.Cancer Res.1962;22:525-548.[64] Liou GY,Storz P.Reactive oxygen species in cancer.Free Radic Res. 2010;44(5):479-496. |
.jpg)
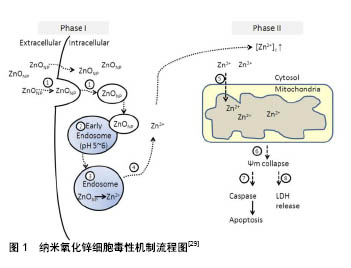
.jpg)