| [1] Ma JJ,Fan SW,Zhang J,et al. Anterior decompression and reconstruction for lumbar burst fractures. Orthop Surg. 2015;7(2):187-188.[2] 颜滨,杨欣建,沈哲,等.手术治疗下腰椎爆裂性骨折合并神经损伤[J].中华创伤杂志,2014,30(6):535-536.[3] 罗一,邓展生,陈静.不同植骨融合方式对胸腰椎爆裂性骨折疗效的影响[J].中国修复重建外科杂,2011,25(11):1302-1307.[4] 李同相,肖睿,官清,等.改良法经病椎植钉复位椎弓根植骨后路钉棒治疗胸腰椎爆裂性骨折[J].中国修复重建外科杂志, 2012,26(5):546-549.[5] 顾华,付建,易难,等.AF椎弓根螺钉内固定系统治疗胸腰椎爆裂性骨折:30个月随访的中远期效果分析[J]. 中国组织工程研究, 2012,16(13):2378-2381.[6] 成红兵,李佳.CD2椎弓根螺钉置入内固定治疗胸腰椎爆裂性骨折[J].中国组织工程研究与临床康复,2010,14(39):7402-7406.[7] 邱满乐,连小峰,李浩,等.经后路前中柱稳定性重建术治疗严重胸腰椎骨折脱位[J].国际骨科学杂志,2014,35(3):1673-1678.[8] 曾忠友,吴鹏,唐宏超,等.椎弓根螺钉联合椎板关节突螺钉固定治疗腰椎骨折[J].中华创伤骨科杂志,2014,16(3):212-217.[9] Allain J.Anterior spine surgery in recent thoracolumbar fractures: An update.Orthop Traumatol Surg Res. 2011;97(5):541-554.[10] Riaz S,Fox R,Lavoie MV,et al.Vertebral body reconstruction for thoracolumbar spinal metastasis--a review of techniques.J Ayub Med Coll Abbottabad.2006;18(1):70-77.[11] 张晓星,邓志龙,苟景跃.前路减压植骨内固定治疗胸腰椎爆裂骨折疗效观察[J].创伤外科杂志,2013,15(3):266-269.[12] 阳建,谢景运,钟坚,等.一期前后路联合手术治疗胸腰椎结核的疗效观察[J].湘南学院学报(医学版),2012,13(3):23-25.[13] 涂强,许建中.胸腰椎前路短节段内固定器的发展与应用[J].创伤外科杂志, 2004,6(2):152-155.[14] Higashino K,Katoh S,Sairyo K,et al.Pseudoaneurysm of the thoracoabdominal aorta caused by a severe migration of an anterior spinal device.Spine J.2008;8(4):696-699.[15] Oskouian RJ,Johnson JP.Vascular complications in anterior thoracolumbar spinal reconstruction. J Neurosurg.2002;96(1 Suppl):1-5.[16] 任朝辉,吕国华,王冰.胸腰椎前路手术手术期并发症及其预防[J].中国脊柱脊髓杂志,2007,17(8):571-574.[17] Dang Y,Yen D,Hopman WM.Postoperative bedrest improves the alignment of thoracolumbar burst fractures treated with the AO spinal fixator.Can J Surg.2009;52(3):215-220.[18] 金大地,陈建庭,瞿东滨,等.胸腰椎前路K形钢板内固定系统的研制及临床初步应用[J].中华外科杂志,2001,39(9):704-707.[19] 陈前芬,肖增明,李世德,等.胸腰椎前路手术并发症分析和对策[J].中国矫形外科杂志,2009,17(7):546-548.[20] Zhou CS,Xu YF,Zhang Y,et al.Biomechanical testing of a unique built-in expandable anterior spinal internal fixation system.BMC Musculoskelet Disord.2014;15:424.[21] Lacey DC,De Kok B,Clanchy FI,et al.Low dose metal particles can induce monocyte/macrophage survival.J Orthop Res. 2009;27(11):1481-1486.[22] Hallab NJ,Jacobs JJ.Biologic effects of implant debris.Bull NYU Hosp Jt Dis.2009;67(2):182-188.[23] Cadosch D,Chan E,Gautschi OP,et al.Titanium IV ions induced human osteoclast differentiation and enhanced bone resorption in vitro.J Biomed Mater Res A.2009;91(1):29-36.[24] 由少华,钱承玉,朱雪涛,等. GB-T 16886.10-2005 医疗器械生物学评价:第10部分刺激与迟发型超敏反应试验[M]. 第二修订版.国家药品监督管理局,济南医疗器械质量监督检验中心, 2005:11-14.[25] 昊平,刘秦玉,由少华,等. GB-T 16886.1-2001 医疗器械生物学评价:第1部分评价与试验[M]. 第二修订版.国家药品监督管理局,济南医疗器械质量监督检验中心,2001:304-309.[26] 陈旭琼,尹庆水,张余,等.镁铝合金最大剂量的致敏试验[J].中国组织工程研究与临床康复,2010,14(16):2899-2902.[27] 林山,黄晓梅,芮钢,等.数字化珊瑚羟基磷灰石人工骨的致敏实验[J].中国组织工程研究,2014,18(25):3961-3965.[28] Ohnishi T,Neo M,Matsushita M,et al.Delayed aortic rupture caused by an implanted anterior spinal device.Case report.J Neurosurg. 2001;95 (2Suppl):253-256.[29] Higashino K,Katoh S,Sairyo K,et al.Pseudoaneurysm of the thoracoabdominal aorta caused by a severe migration of an anterior spinal device.Spine J.2008;8(4):696-699.[30] 徐彦芳,孙进,周初松,等.全内置式可膨胀型脊柱前路内固定系统研制的解剖学基础[J].实用医学杂志, 2013,29(3):352-354.[31] Kokubo T,Kim HM,Kawashita M.Novel bioactive materials with different mechanical properties. Biomaterials. 2003;24(13):2161-2175.[32] 王周成,黄龙门.金属基生物活性羟基磷灰石涂层材料的研究进展[J].硅酸盐通报,2006,25(1):57-62.[33] Samar JK,Abhilasha B,Himesh AB.Nanocrystalline calcium phosphate ceramics in biomedical engineering.Mater Sci Eng C.2007;27:441-449.[34] 韩雪,宋九余,华泽权,等.阳极氧化处理后的新型钛合金体外诱导实验及对成骨细胞早期附着的影响[J].实用口腔医学杂志, 2007,23(6):781-784.[35] Tatiani A,Donato G,Luciano H,et al.Cytotoxicity study of some Ti alloys used as biomaterial. Mater Sci Eng C. 2009;29:1365-1369.[36] Magnusson B,Kligman AM.The identification of contact allergens by animal assay.The guinea pig maximization test.J Invest Dermatol.1969; 52(3):268-276.[37] Sato Y,Katsumura Y,Ichikawa H, et al. A modified technique of guinea pig testing to identify delayed hypersensitivity allergens.Contact Dermatitis. 1981;7(5):225-237.[38] Dvorak HF, Dvorak AM, Simpson BA, et al. Cutaneous basophil hypersensitivity. II. A light and electron microscopic description.J Exp Med.1970;132(3):558-582. |
.jpg)
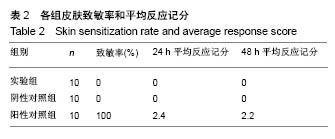
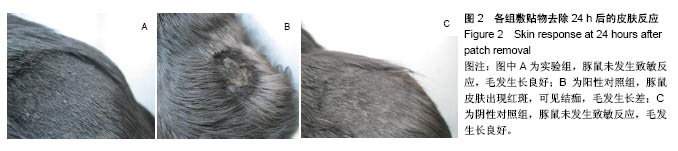
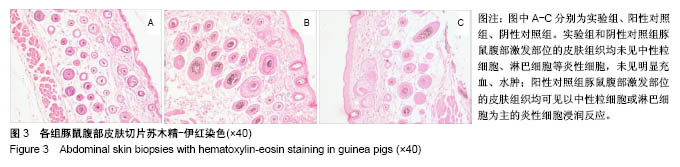
.jpg)
.jpg)
.jpg)