中国组织工程研究 ›› 2024, Vol. 28 ›› Issue (2): 280-287.doi: 10.12307/2023.679
• 组织构建综述 tissue construction review • 上一篇 下一篇
免疫细胞及相关细胞因子在骨关节炎发病及治疗中的作用
孟志成1,乔卫平2,赵 阳1,刘洪飞1,李凯杰2,马 博2
- 1河南中医药大学,河南省郑州市 450000;2河南中医药大学第一附属医院骨伤科,河南省郑州市 450000
-
收稿日期:2022-09-03接受日期:2022-10-20出版日期:2024-01-18发布日期:2023-06-30 -
通讯作者:乔卫平,副主任医师,河南中医药大学第一附属医院骨伤科,河南省郑州市 450000 -
作者简介:孟志成,男,1996年生,山东省滨州市人,汉族,硕士,主要从事中西医结合防治骨伤疾病研究。
Effects of immune cells and related cytokines in the pathogenesis and treatment of osteoarthritis
Meng Zhicheng1, Qiao Weiping2, Zhao Yang1, Liu Hongfei1, Li Kaijie2, Ma Bo2
- 1Henan University of Traditional Chinese Medicine, Zhengzhou 450000, Henan Province, China; 2Department of Orthopedics and Traumatology, The First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou 450000, Henan Province, China
-
Received:2022-09-03Accepted:2022-10-20Online:2024-01-18Published:2023-06-30 -
Contact:Qiao Weiping, Associate chief physician, Department of Orthopedics and Traumatology, The First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou 450000, Henan Province, China -
About author:Meng Zhicheng, Master, Henan University of Traditional Chinese Medicine, Zhengzhou 450000, Henan Province, China
摘要:
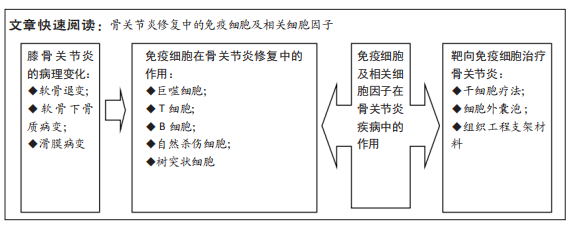
文题释义:
骨关节炎:是一种退行性关节疾病,可逐渐导致关节功能丧失甚至身体残疾,骨关节炎最常累及的关节是膝关节,主要影响老龄人群及肥胖人群,临床症状为膝关节疼痛、肿胀、僵硬和活动能力下降,主要病理表现以软骨退变为主,进而累及滑膜、软骨下骨及关节内其他组织发生退化改变。免疫细胞:参与免疫应答或者与免疫应答有关的细胞统称为免疫细胞,包括淋巴细胞、单核吞噬细胞、巨噬细胞、自然杀伤细胞等。在免疫应答过程中,起核心作用的是淋巴细胞,其能接受抗原刺激而活化、增殖及分化。发生特异性免疫反应的淋巴细胞,称为抗原特异性淋巴细胞或免疫活性细胞,他们表面有抗原特异性受体,能特异性识别抗原。巨噬细胞和自然杀伤细胞虽然可以识别抗原,但对抗原的识别并不是特异的。
目的:综述免疫细胞及相关细胞因子在骨关节炎发病及治疗中的作用,为今后骨关节炎的研究和防治提供新的思路。
方法:以“骨关节炎,膝,巨噬细胞,T细胞,B细胞,自然杀伤细胞,树突状细胞,细胞因子,炎症因子,免疫细胞” “osteoarthritis,knee,macrophages,T cells,B cells,natural killer cells,dendritic cells,cytokines, inflammatory factors,immune cells”为检索词在中国知网、万方、维普和PubMed 和Web of Science数据库中检索已发表的相关文献,阅读标题和摘要进行初筛,在阅读全文后,最后选取98篇文献进行综述。
结果与结论:①既往研究认为骨关节炎发病与软骨磨损相关,近年来研究认为骨关节炎是免疫细胞广泛参与的一种慢性炎性状态,随着对骨关节炎发病机制的深入研究,学者们认为骨关节炎的发病机制是由早期先天免疫反应驱动的,这种免疫反应会逐步催化退行性变,最终导致关节微环境的改变。②各种免疫细胞和细胞因子是影响骨关节炎修复的关键因素,其中巨噬细胞、自然杀伤细胞参与滑膜炎症反应,T细胞免疫反应参与骨关节炎软骨的降解,并加重骨关节炎的病情。③免疫细胞分泌的白细胞介素1β、白细胞介素6、肿瘤坏死因子α、白细胞介素17和白细胞介素37等炎性细胞因子在骨关节炎的病理生理中发挥重要作用,其中白细胞介素1β是造成关节软骨破坏最重要的炎性因子。④在骨关节炎病早期阶段对免疫学危险因素进行评估,能够早期有效地进行治疗,可显著降低与骨关节炎相关的残疾、发病率和成本。⑤目前干细胞及其来源外泌体、生物材料进行免疫调节治疗骨关节炎的效果已在不同的实验模型中得到证实,但将其用于临床实践之前仍有大量的实验工作要做,随着新治疗靶点的发现,针对靶点进行相关治疗将为临床骨关节炎修复带来新的希望。
https://orcid.org/0000-0003-0209-2212 (孟志成)
中国组织工程研究杂志出版内容重点:组织构建;骨细胞;软骨细胞;细胞培养;成纤维细胞;血管内皮细胞;骨质疏松;组织工程
中图分类号:
引用本文
孟志成, 乔卫平, 赵 阳, 刘洪飞, 李凯杰, 马 博. 免疫细胞及相关细胞因子在骨关节炎发病及治疗中的作用[J]. 中国组织工程研究, 2024, 28(2): 280-287.
Meng Zhicheng, Qiao Weiping, Zhao Yang, Liu Hongfei, Li Kaijie, Ma Bo. Effects of immune cells and related cytokines in the pathogenesis and treatment of osteoarthritis[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(2): 280-287.
2.1.1 软骨退变 骨性关节炎最早期的病理改变即发生在关节软骨。关节软骨是一种特殊的组织,无血管和淋巴以及神经支配,主要由软骨细胞和细胞外基质组成,细胞较少而基质丰富。软骨细胞外基质的基本成分主要有胶原蛋白、纤连蛋白和层粘连蛋白、糖胺聚糖和生物活性生长因子等,其不仅能维持软骨细胞的生存环境,对软骨细胞起支撑和营养作用,同时还可作为软骨细胞与外界进行信号交换的场所,对维持软骨细胞稳态具有重要作用。
研究发现,骨性关节炎发生后软骨细胞大量凋亡,主要是由于关节损伤部位的基质成分丢失及基质结构破坏,阻碍了软骨细胞的正常代谢功能,使软骨受损范围及深度逐渐扩大,最终关节软骨全层受损。关节软骨破坏的病理过程中有多种炎性递质参与软骨基质降解。肿瘤坏死因子α是公认的一种参与免疫及炎症反应的细胞因子,与白细胞介素1、白细胞介素6及白细胞介素8一起被认为是前炎性细胞因子,而关节软骨持续的炎症反应是造成关节软骨损伤的主要原因。人们认为肿瘤坏死因子α通过触发免疫反应、增加或减少其他炎性细胞因子的产生,在复杂的免疫网络中发挥“主调节剂”的作用[4]。体外实验证实,白细胞介素1及肿瘤坏死因子α促进白细胞介素6合成,同时白细胞介素6也促进白细胞介素1的表达。这些炎症递质的相互作用导致关节软骨加速破坏,同时白细胞介素1与白细胞介素6共同促进前列腺素、一氧化氮及环氧合酶合成,导致关节疼痛和肿胀[5]。白细胞介素1β和肿瘤坏死因子α可以通过核转录因子κB依赖性上调原代关节软骨细胞中ASIC1a的表达来增强酸中毒诱导的细胞毒性[6]。白细胞介素6被认为是炎症反应中生物效应的放大因子,其作为核因子κB受体活化因子配体(receptor activator of nuclear factor-κ B ligand,RANKL)表达的受体激活剂,激活黏附分子,将白细胞募集到骨关节处,破坏细胞外基质[7]。关节软骨促炎细胞因子水平与骨关节的发生和发展密切相关。
基质金属蛋白酶是水解细胞外基质的一种蛋白水解酶,在研究骨关节炎软骨破坏的机制中,基质金属蛋白酶被认为是软骨细胞外基质降解的主要酶[8]。基质金属蛋白酶13是促使细胞外基质降解的主要酶,它能不可逆地降解Ⅱ型胶原,从而造成关节软骨的破坏[9]。基质金属蛋白酶3可受到多种因素的影响,如白细胞介素1β、肿瘤坏死因子α、Runt核转录因子2可以诱导基质金属蛋白酶3的表达[10-11]。基质金属蛋白酶13启动子区域的低甲基化也会诱导其表达,但可以通过抑制组蛋白乙酰化来降低其表达[12]。蛋白聚糖降解率的提高对于骨关节炎的软骨破坏至关重要。最近发现,基质金属蛋白酶17与聚蛋白多糖酶4的激活有关[13]。聚蛋白多糖酶在骨关节炎病变过程中起重要作用,免疫组化结果发现聚蛋白多糖酶4在磨损的关节软骨中高表达,白细胞介素1能够使骨关节炎软骨的聚蛋白多糖酶4 mRNA表达上调,加速软骨破坏,对此酶的抑制不仅有可能防止骨关节炎的发生,而且有可能控制骨关节炎的进展[14-15]。另外,研究发现关节失稳后出现的异常机械摩擦力也是聚蛋白多糖酶活性上调的原因之一,维护关节的稳定性并同时控制炎症因子白细胞介素1的活性才能更好地阻止骨关节炎的发生和发展[16]。
2.1.2 软骨下骨质病变 软骨下骨位于软骨下方,包括关节软骨的钙化层(软骨下骨板)和一层薄薄的骨皮质层(骨小梁)。软骨下骨板将松质骨及关节软骨分隔开,可以为关节软骨提供坚硬的支撑,还具有维持关节弹性及减震作用。很多研究发现,软骨下骨在骨关节炎进程中起着重要的作用,骨关节炎早期或进展阶段,软骨下骨的骨改建活跃,骨转换增强导致骨体积增加,骨矿物质密度降低[17]。而骨关节炎晚期阶段,骨转换减少,骨吸收减弱,而骨形成相对增加,影像学上表现为软骨下骨板骨质硬化[18],导致载荷传导发生了变化,使力学刚度下降,丧失了保护上方关节软骨的作用[19]。研究证明来自骨关节炎硬化骨组织的间充质干细胞/骨祖细胞也具有低矿化表型[20]。因此,软骨下骨板的硬化、软骨下松质骨的骨量丢失以及骨矿含量的下降都是骨关节炎病变的起始因素[21]。
2.1.3 滑膜病变 滑膜的炎症在骨关节炎中起重要作用,可能是骨关节炎的超早期表现,滑膜炎症和滑膜相关的炎性递质和细胞因子、蛋白诱导关节软骨的降解和破坏,促进了骨关节炎的发展。滑膜炎的特征在于成纤维细胞样滑膜细胞的增殖和巨噬细胞募集,导致滑膜内层增生和增厚,增生增厚的滑膜会分泌过多液体造成关节疼痛及关节肿胀等症状。研究表明,滑膜成纤维细胞表达白细胞介素6,这是一种与骨关节炎疼痛和放射学进展独立相关的细胞因子[22-23],但位于滑膜亚层的 CD34+CD90+成纤维细胞表达的白细胞介素6比滑膜内层中的 CD34-CD90-成纤维细胞要多得多[24]。其他促炎细胞因子,包括 白细胞介素1β、白细胞介素2、白细胞介素6、白细胞介素8、白细胞介素12、干扰素γ和肿瘤坏死因子α在骨关节炎患者滑液中也显著升高。还有研究表明,骨关节炎发病可能与滑膜内特异表达的基因诱导细胞外基质的降解有关,基质金属蛋白酶1和基质金属蛋白酶9可能是用于治疗骨关节炎的有效分子靶标[25]。
在膝骨关节炎患者的滑液中,白细胞介素18与前列腺素E2水平密切相关[26]。前列腺素E2被认为是骨关节炎性疼痛的主要原因,前列腺素E2参与增强由促炎细胞因子诱导的聚集蛋白聚糖酶和基质金属蛋白酶13的产生。骨关节炎滑膜中,白细胞介素18促进软骨细胞增殖和环氧合酶2、诱导型一氧化氮合酶和基质金属蛋白酶的表达,并促进白细胞介素6的产生,进一步支持促分解代谢环境。与健康对照组相比,骨关节炎患者的补体因子在涉及骨关节炎的主要组织(包括软骨、骨和滑膜)中高度表达[27-28]。此外,与健康供体相比,骨关节炎患者滑膜中补体效应物的表达更高。此外,C5和C6缺乏对骨关节炎动物模型中滑膜炎和软骨损伤的发展具有保护作用,并且补体激活与细胞外基质降解蛋白和炎症递质的产生增加有关[27]。这些数据表明补体激活不仅在滑膜炎中而且在骨关节炎的发展和进展中都起作用,因此它可能代表一个潜在的治疗靶点。
因此,骨关节炎是以关节结构的病理变化为主要特征,包括软骨侵蚀、滑膜炎症和软骨下硬化伴骨赘形成,主要由多种致病因素触发关节软骨正常内稳态丧失和结构变化导致的。深入了解骨性关节炎的发病机制和致病介质,能够为骨关节炎的预防、诊断和治疗提供重要依据。
2.2 免疫细胞在骨关节炎修复中的作用 研究发现参与骨关节炎修复的免疫细胞主要包括巨噬细胞、T细胞、B细胞、自然杀伤细胞和树突状细胞等[29],以下主要对这几种细胞进行探讨。
2.2.1 巨噬细胞 受微环境刺激,巨噬细胞可极化成M1型巨噬细胞和M2型巨噬细胞,发挥不同的生理作用[30],见图4。
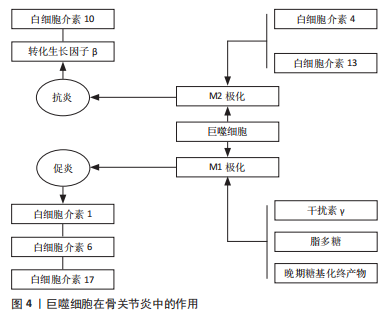
干扰素γ在自然杀伤细胞和Th1细胞活化后释放,将浸润的巨噬细胞极化为M1型巨噬细胞[31]。干扰素γ、脂多糖和晚期糖基化终产物可定向诱导巨噬细胞向M1型巨噬细胞分化,通过激活Toll样受体信号通路,分泌一系列促炎细胞因子和趋化因子,触发各种免疫反应,包括杀死病原体和引发炎症,最后通过级联反应,进一步促进炎症的发展。M1型巨噬细胞表面的模式识别受体和Toll 样受体可识别关节腔内的损伤相关分子模式,从而激活下游炎症信号通路,如核转录因子κB 信号通路,诱导释放大量的促炎细胞因子;另一方面,M1型巨噬细胞还可以诱导一氧化氮的产生,并表达高水平的共刺激分子(如CD80和CD86)和组织相容性复合物分子,对初始T细胞活化起重要作用。
研究表明,来源于M1型巨噬细胞的炎性细胞因子不仅诱导软骨细胞破坏,而且通过促进蛋白水解酶的产生来抑制Ⅱ型胶原和蛋白聚糖合成,是骨软骨修复的主要障碍[32]。研究表明,mTORC1通过驱动M1型巨噬细胞的极化来促进肥大软骨细胞的发育和成熟[33]。M1型巨噬细胞分泌的炎性因子不仅促进软骨细胞向肥大软骨细胞分化,还能通过诱导关节软骨细胞终末分化,降解基质蛋白多糖和关节软骨[34]。FAHY等[35]研究发现M1型巨噬细胞分泌的促炎因子能诱导软骨细胞退化,主要表现为下调Ⅱ型胶原和聚集蛋白聚糖的合成。总之,M1型巨噬细胞不利于软骨修复。
巨噬细胞能够被Th2细胞释放的白细胞介素4和白细胞介素13等诱导活化形成M2型巨噬细胞[36]。然后,M2型巨噬细胞可以产生并释放大量的抗炎细胞因子、免疫抑制因子和促进软骨分化的细胞因子,如白细胞介素10和转化生长因子β,在减少炎症反应和促进软骨修复方面发挥重要作用[37-39],血管内皮生长因子在促进基质成分胶原和糖胺多糖合成方面发挥重要作用,从而促进组织再生。HU等[40]研究证明槲皮素通过抑制软骨细胞凋亡、调节滑膜巨噬细胞向M2巨噬细胞的极化,为软骨细胞创造促软骨修复环境而发挥软骨保护作用。SESIA等[41]研究发现M2型巨噬细胞和骨髓间充质干细胞共培养能促进骨髓间充质干细胞成软骨分化。DAI等[42]研究表明M2型巨噬细胞通过分泌转化生长因子β1和转化生长因子β3促进软骨的修复。另外白细胞介素10不仅具有抗炎作用,近期研究表明白细胞介素10促进成软骨分化的能力也很强。这些结果表明,M2型巨噬细胞通过分泌多种细胞因子促进组织的修复,是组织再生和修复的关键细胞。
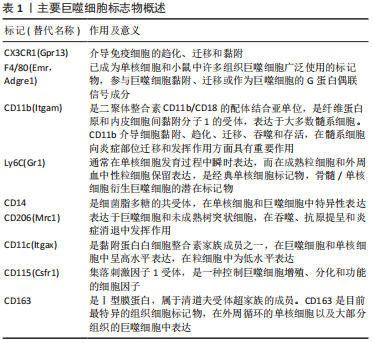
一项对来自两个不同队列的184例膝骨关节炎患者的研究发现,滑液中巨噬细胞标记物CD14和CD163水平与骨赘严重程度相关,而滑液CD14和血清CD163水平与关节间隙狭窄的严重程度相关,滑液和血清中的CD14水平与膝关节症状的严重程度相关[43]。滑液中CD11c+与 CD206+巨噬细胞或CD86+与CD163+巨噬细胞的比率与KL分级和患者膝骨关节炎的严重程度相关[44]。骨关节炎滑膜中的促炎巨噬细胞显示基质金属蛋白酶(包括基质金属蛋白酶1、基质金属蛋白酶3、基质金属蛋白酶13和基质金属蛋白酶9)、聚集蛋白聚糖酶(包括ADAMTS4和ADAMTS5)和环氧合酶2上调,导致关节退化[45]。CD68+巨噬细胞还通过吞噬胶原蛋白片段并将其呈递给CD4+T细胞,从而导致关节Ⅱ型胶原蛋白的丢失[46]。表1列出了可用于识别巨噬细胞的标志物。
抗炎巨噬细胞还表达甘露糖受体MRC1和MRC2,它们与胶原蛋白结合并促进其内化和溶酶体降解,恢复了关节中的细胞外基质稳态并改善了软骨破坏[47]。重要的是,Ⅱ型胶原蛋白有助于维持巨噬细胞中抗炎基因以及促软骨细胞因子的表达[48]。
巨噬细胞表型研究还确定了替代激活的巨噬细胞亚群(既不富含促炎标志物也不富含抗炎标志物的巨噬细胞),它们更有可能参与炎症的愈合[49]。事实上,骨关节炎滑膜巨噬细胞并不完全符合与经典促炎或抗炎表型相对应的表面标志物表达谱[50],scRNA-seq研究检测到骨关节炎滑膜中有异质巨噬细胞类型,包括免疫调节(表达SEPP1,FOLR2,STAB1,TXNIP和CD169)和促炎(表达CCL3,CCL4,白细胞介素1β和肿瘤坏死因子)巨噬细胞亚群。这种与典型的促炎或抗炎表型不一致的免疫调节群体表现出的基因表达谱表明吞噬活性和免疫抑制活性增强。
由于巨噬细胞的动态平衡在疾病发展过程中的重要性,巨噬细胞已经成为骨关节炎病理机制和治疗策略的研究热点。需要更好地了解巨噬细胞亚群及其在骨关节炎中的作用,病理性巨噬细胞亚群的鉴定可能为减少滑膜炎和组织损伤提供一个很好的机会。
2.2.2 树突状细胞 树突状细胞是人体中具有较强的处理、加工抗原信息的细胞,因为这种细胞成熟时会有很多伪足,像树突一样,故称为树突状细胞。树突状细胞是目前所知抗原提呈功能最强的抗原提呈细胞,它的数量和活性状态会直接影响免疫应答过程,人体内大部分树突状细胞处于非成熟状态,表达低水平的共刺激因子和黏附因子,体外激发同种混合淋巴细胞增殖反应的能力较低,未成熟树突状细胞不仅可以促进Treg细胞增殖,还可以通过分泌白细胞介素10促进间充质干细胞向软骨细胞分化,有助于软骨修复[51]。研究发现,一旦Toll样受体被病原相关分子模式刺激,未成熟树突状细胞历经一个快速的成熟过程,成熟树突状细胞快速失去细胞内吞活性,上调细胞表面MHC分子及共刺激分子的表达,分泌炎症因子,加重滑膜炎并促进软骨退化[52-55]。此外,成熟树突状细胞激活Th1和Th17细胞,分泌促炎因子,促进软骨退化[56-57]。
2.2.3 自然杀伤(NK)细胞 NK细胞是一种天然的效应淋巴细胞,它能介导对应激、恶性和病毒感染细胞的有效免疫反应。与B细胞和T细胞相比,它们可以立即起作用,不需要任何启动或抗原呈递。NK细胞通过释放细胞因子和趋化因子以及与其他免疫细胞的相互作用发挥调节作用。最近关于膝骨关节炎危险因素的研究表明,滑膜炎可能是膝骨关节炎的独立病因素,也是影响膝骨关节炎发病和进展的独立驱动因素[58]。
NK细胞是骨关节炎患者增生滑膜的主要免疫细胞亚群之一,在骨关节炎的发生和发展中起着至关重要的作用[59]。NK细胞表面标志的特异性是相对的,常用检测NK细胞的标记有CD16,CD56,CD57,CD59,CD11b,CD94和LAK-1。彭志伟[60]通过CD16,CD56分子的表达将NK细胞分为CD16+CD56+NK细胞和CD16-CD56+NK细胞两种类型,CD16+CD56+NK细胞可以对靶细胞发挥非特异杀伤效应,具备更强的细胞毒活性,而CD16-CD56+NK细胞在单核细胞激活后产生非常丰富的趋化因子的作用,但不具备抗体依赖性细胞介导的细胞毒性作用。膝骨关节炎患者外周血中CD16+CD56+NK细胞比例明显高于对照组,而CD16-CD56+NK细胞无明显变化,说明外周血中NK细胞的主要亚群是以杀伤功能为主的CD16+CD56+NK细胞 ,并认为NK细胞在膝骨关节炎中的作用可能与VLA家族的黏附作用有关[57]。有研究表明,CD16-CD56+NK细胞在白细胞介素2的作用下可以向CD16+CD56+NK细胞转化[61],推测关节损伤后引发白细胞趋化,释放白细胞介素2,促使CD16-CD56+NK细胞向CD16+CD56+NK细胞转化,对关节内结构细胞组织杀伤增强,引发并加剧了关节结构的退变。
2.2.4 T细胞 T细胞通过分泌细胞因子和生长因子促进细胞外基质降解和重塑[62]。骨关节炎患者滑液中的T细胞数量显著增加。此外,滑膜尤其是血管周围有明显的T细胞(主要是CD4+T细胞)浸润[63]。抗原活化的CD4+T细胞分为4种亚型:Th1,Th2,Th17和Tregs。Th1主要分泌白细胞介素2、干扰素γ和肿瘤坏死因子α,主要介导细胞免疫应答,促进细胞毒T细胞(CTL)的杀伤作用,激活巨噬细胞杀灭细胞内病原体(包括病毒和细菌)等。Th2细胞主要分泌白细胞介素4、白细胞介素5、白细胞介素6、白细胞介素10和白细胞介素13,主要调节体液免疫反应,也参与B细胞的活化。巨噬细胞和树突状细胞分泌细胞因子如白细胞介素4,通过各种途径促进Th2细胞分化[64]。
此外,Th17产生白细胞介素17,白细胞介素17是一种炎性细胞因子,已发现其作用于软骨细胞并阻断蛋白多糖的产生,从而抑制软骨修复[65]。值得注意的是,白细胞介素17可以吸引中性粒细胞和巨噬细胞进入受损组织,以帮助宿主对抗感染。Th17还可促进软骨细胞中基质金属蛋白酶1、3和13的表达,同时抑制Ⅱ型胶原、蛋白多糖的表达,这些均可促进软骨破坏[66-67]。Tregs是调节性T细胞,主要包括两个亚群,一种是CD4+CD25+调节性T细胞,具有抗炎特性[68];另一种为CD8+调节性T细胞,其主要包括CD8+CD28-调节性T细胞和CD8+ CD28-限制性的调节性T细胞。研究表明,活化的Tregs促进抗炎分子的分泌,包括白细胞介素10、转化生长因子β和吲哚胺2,3-双加氧化酶,并抑制白细胞介素6分泌,从而维持再生微环境的稳态并间接促进组织再生[69]。此外,T细胞负责激活巨噬细胞和B细胞,CD4+T细胞增加与巨噬细胞浸润和基质金属蛋白酶9表达有关,可加速软骨退化[70]。
2.2.5 B细胞 作为一种主要的免疫细胞,B细胞分泌的自身抗体可促进类风湿关节炎等自身免疫病的发生和发展。B细胞表面的膜免疫球蛋白、Fc受体、补体受体、EB病毒受体和小鼠红细胞受体是B细胞的重要表面标志,其中以膜免疫球蛋白为B细胞所特有,是鉴定B细胞可靠的指标。B细胞表面较特异的CD分子有CD19,CD20,CD21,CD22和CD23等。研究表明,B细胞分泌多种促炎因子,包括白细胞介素1β、白细胞介素6和肿瘤坏死因子α,以诱导软骨细胞死亡和软骨基质破坏[71-72]。但近期研究发现,体内也在存在一类具有特殊功能的B细胞亚群,它们参与免疫应答的调节、介导免疫耐受、发挥免疫抑制作用,这种具有保护功能的B细胞亚群即调节性B细胞[73]。调节性B细胞通过分泌白细胞介素10而抑制CD4+T细胞分泌干扰素γ和肿瘤坏死因子α的表达,减轻炎症反应,从而间接促进软骨修复[74]。
综上所述,大量的证据表明免疫系统参与骨关节炎发生发展,免疫反应越来越多地被认为是影响软骨修复的关键因素,它对修复过程既有消极的调节作用,也有积极的调节作用。不同的免疫细胞被募集以产生大量的促炎因子和组织修复因子。因此,需要对关节炎症微环境进行多维调控,进一步研究区分免疫细胞亚群,确定哪些细胞可以作为修复软骨损伤的靶细胞,使其有利于软骨修复。
2.3 靶向免疫细胞治疗骨关节炎 骨关节炎进程涉及许多病理变化,包括滑膜细胞功能改变、软骨细胞凋亡和肥大以及免疫细胞激活,所有这些都可导致炎性关节微环境的形成,并对软骨修复产生重大影响。促炎因子不仅影响工程化软骨与周围正常软骨的整合,而且抑制间充质干细胞向软骨细胞的分化。对移植物的异常免疫反应可能导致慢性炎症及纤维化等,相反,适当免疫调节可以促进软骨修复。因此,免疫反应在软骨修复中是一把双刃剑。为创造促进软骨修复的微环境,免疫细胞已成为关键的治疗靶点,见表2。
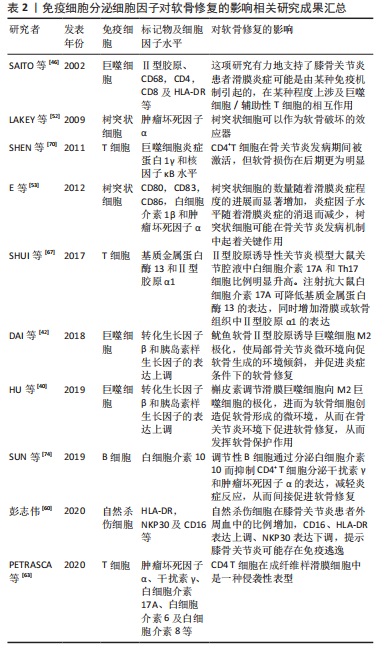
2.3.1 干细胞疗法 间充质干细胞已被证明具有广泛的免疫调节特性,它们可以抑制免疫细胞的迁移和活化以及炎症因子的产生。通过调节免疫应答,间充质干细胞可以积极地改变损伤微环境,促进其向软骨方向分化。间充质干细胞可以抑制中性粒细胞活化及基质金属蛋白酶的过度表达[75]。研究还证实,基于间充质干细胞的组织工程软骨可以促进巨噬细胞极化为M2表型[76],使巨噬细胞表现出抗炎特性。间充质干细胞除了能抑制T细胞和B细胞的增殖和活化外,还能促进Treg和Breg细胞的增殖[77]。炎症因子和低氧等预处理可增强间充质干细胞的免疫调节作用,从而优化治疗效果。
VOSKAMP等[78]研究表明肿瘤坏死因子α预处理可以促进骨髓间充质干细胞的增殖、迁移和成软骨能力,有利于组织再生,进一步表明这种作用可能是过调节 SOX11水平和 Wnt/β-catenin 信号传导而实现的。有趣的是,该作者用另一种促炎细胞因子白细胞介素1β进行预处理并没有增加骨髓间充质干细胞的软骨形成能力,这表明促软骨形成作用对肿瘤坏死因子α而言具有特异性。MATSUMURA等[79]应用白细胞介素1β预处理滑膜间充质干细胞14 d,结果发现滑膜间充质干细胞数量呈浓度依赖性增加;白细胞介素1β预处理滑膜间充质干细胞在软骨形成培养基中培养 21 d时,成软骨能力显著提高。当与正常氧环境中生长的脂肪间充质干细胞相比,在低氧环境中培养的脂肪间充质干细胞可以促进CD4+T细胞向Tregs转化[80]。值得注意的是,各种干细胞亚群已被证明具有不同的免疫调节作用。例如,CD146+脂肪间充质干细胞在关节腔内注射早期阶段显示出优越的抑制炎症特性[81];磁珠分选后的CD105+滑膜间充质干细胞具有更强的成软骨能力[82]。
2.3.2 细胞外囊泡 随着研究的进展,越来越多的证据表明间充质干细胞主要通过旁分泌机制来发挥治疗作用,而细胞外囊泡是间充质干细胞的重要旁分泌成分,其通过携带生物活性分子和基因调控信息来促进细胞间交流。
WOO等[83]研究探讨了人脂肪干细胞来源细胞外囊泡治疗骨关节炎的潜力及其机制,通过基于切向流过滤的多级过滤系统从人脂肪干细胞培养上清液中分离细胞外囊泡,体外实验结果发现人脂肪干细胞来源细胞外囊泡促进了白细胞介素1β诱导的人软骨细胞的增殖和迁移,而且通过增加Ⅱ型胶原的合成和降低基质金属蛋白酶1、基质金属蛋白酶3、基质金属蛋白酶13和聚集蛋白聚糖酶2的表达来维持软骨细胞基质。在碘乙酸单钠诱导的骨关节炎大鼠中,关节内注射人脂肪干细胞来源细胞外囊泡可显著减缓骨关节炎进展,保护软骨不发生变性。
WANG等[84]评估了正常CBA小鼠和MRL/MpJ小鼠成软骨祖细胞分泌的细胞外囊泡对小鼠软骨细胞增殖和迁移的影响并进行体内实验验证,体外结果显示两种细胞外囊泡均能刺激软骨细胞增殖和迁移,而且MRL/MpJ小鼠成软骨祖细胞来源细胞外囊泡发挥更强的作用;在内侧半月板失稳模型(一种手术诱导的骨关节炎小鼠模型)中,关节内注射两种小鼠成软骨祖细胞来源细胞外囊泡,体内结果表明注射后均能减轻骨关节炎症状,且MRL/MpJ小鼠成软骨祖细胞来源细胞外囊泡显示出更好的治疗效果。生物信息学分析结果表明,差异表达的外泌体miRNA参与了多个生物学过程,前20个差异表达的外泌体 miRNA将骨关节炎修复与AMPK信号传导、自噬调节和胰岛素信号传导等过程联系起来。值得注意的是,miR-221-3p在MRL/MpJ小鼠成软骨祖细胞来源细胞外囊泡中高度富集,并且在体外用miR-221-3p 抑制剂治疗显著降低了细胞外囊泡诱导的软骨细胞增殖和迁移。
最近的研究数据表明,间充质干细胞衍生的外泌体和间充质干细胞具有类似的免疫调节功能。在炎性关节炎模型中,间充质干细胞衍生的外泌体在体内抑制炎症甚至比间充质干细胞更有效[85]。凌华军等[86]研究表明骨髓间充质干细胞来源外泌体可以减弱白细胞介素1β对软骨细胞的损伤作用,促进损伤关节软骨形态恢复及Ⅱ型胶原纤维α1表达,抑制基质金属蛋白酶13表达;体内实验同样证实外泌体可以缓解碘乙酸对骨关节软骨的破坏作用,延缓骨关节炎的发生与发展。据报道,脂肪间充质干细胞来源外泌体与滑膜成纤维细胞共培养时,外泌体下调了炎症因子如白细胞介素6及肿瘤坏死因子α水平,而上调了白细胞介素10水平[87]。另一项研究使用生物信息学分析和体内实验表明,脂肪间充质干细胞来源外泌体可诱导M2巨噬细胞极化,抑制T细胞增殖,促进Th1细胞向Th2细胞转化,促进Treg细胞扩增,促进骨关节炎软骨组织修复[88]。间充质干细胞衍生细胞外囊泡通过抑制M1巨噬细胞浸润和促进M2巨噬细胞极化,促进软骨细胞增殖和细胞外基质合成,同时降低炎性细胞因子如白细胞介素1β和肿瘤坏死因子α的表达,改善关节微环境,促进软骨修复[89]。
外泌体作为遗传信息的载体在细胞间通讯中发挥作用,在细胞之间转移 microRNA(miRNA或miR),并已被研究作为治疗分子的传递载体。CHEN等[90]发现过表达 miR-150-5p 的间充质干细胞衍生的外泌体通过抑制滑膜细胞增生和血管生成来减少关节破坏。JIANG等[91]体外实验结果表明,人脐带沃顿氏胶间充质干细胞可促进骨髓间充质干细胞的迁移、增殖和软骨细胞的增殖,还发现人脐带沃顿氏胶间充质干细胞可以促进巨噬细胞向M2表型极化。兔膝骨软骨缺损修复模型结果证实,人脐带沃顿氏胶间充质干细胞通过调节关节腔的微环境,促进巨噬细胞向M2表型极化和抑制炎症反应,增强脱细胞软骨细胞外基质支架的作用,促进骨软骨再生。
microRNA测序证实人脐带沃顿氏胶间充质干细胞含有许多可促进透明软骨再生的miRNA。ZHANG等[92]证明了骨髓间充质干细胞衍生的外泌体可以减轻软骨损伤,减少骨赘形成和滑膜巨噬细胞浸润,抑制 M1巨噬细胞产生并促进M2巨噬细胞生成。在滑液中,促炎细胞因子白细胞介素1β、白细胞介素6和肿瘤坏死因子α的表达水平降低,抗炎细胞因子白细胞介素10的释放增加;在体外,用外泌体处理的巨噬细胞能够保持软骨细胞的软骨形成特征并抑制肥大。缺氧可以改变间充质干细胞衍生细胞外囊泡的生物学功能。ZHANG等[93]使用体外和体内骨关节炎模型,验证了缺氧预处理的间充质干细胞衍生细胞外囊泡比常氧预处理的间充质干细胞衍生细胞外囊泡更大程度促进了软骨细胞的增殖和迁移并抑制了软骨细胞凋亡。此外,该研究发现缺氧改变了间充质干细胞衍生细胞外囊泡中的 microRNA 表达,并鉴定出4种差异表达的 microRNA:hsa-miR-181c-5p,hsa-miR-18a-3p,hsa-miR-376a-5p和hsa-miR-337-5p。
生物信息学分析表明,低氧预处理可能通过 miR-18-3p/JAK/STAT 或miR-181c-5p/MAPK 信号通路刺激软骨细胞增殖和迁移,抑制软骨细胞凋亡,从而促进软骨修复。因此,缺氧预处理的细胞外囊泡可能是治疗骨关节炎的一种新方法。
近年来,外泌体作为药物载体引起了广泛关注。将外泌体与抗炎药姜黄素混合,具有显著的抗炎作用,减少了炎症相关细胞因子的表达[94]。此外,由于M2巨噬细胞明显促进软骨修复并靶向损伤部位,M2型巨噬细胞来源外泌体可能是继间充质干细胞来源外泌体之后的另一研究热点[95]。
2.3.3 组织工程支架材料 使用生物材料作为支架来运输免疫调节细胞、蛋白质或药物可能是软骨修复的有效方法。材料的物理性质,包括硬度、形状、表面性质和孔径大小,可以引发不同的免疫反应。生物材料可广泛地分为合成材料如聚乳酸-乙醇酸(PLGA)和聚己内酯或天然生物材料如细胞外基质、胶原、弹性蛋白、透明质酸和海藻酸。研究发现软骨细胞外基质通过诱导M2巨噬细胞极化促进骨髓间充质干细胞的迁移、增殖和软骨分化,从而成功修复软骨[96]。聚合物材料具有高度可塑性,其形态、结构和降解速率可针对特定组织进行预先设计,具有可控性、可重复性和良好的力学性能,在软骨组织工程中具有良好的应用前景,但其生物活性低、炎症反应重,不利于与宿主组织结合。因此,大量研究尝试联合具有免疫调节作用的材料或药物促进软骨修复。已发现负载白细胞介素4的水凝胶支架可显著限制炎症因子的产生,并通过促进M2巨噬细胞极化来帮助组织修复[97]。富血小板血浆是一种自体血液衍生产品,PAN等[98]研究表明富血小板血浆-明胶-甲基丙烯酰水凝胶参与巨噬细胞的免疫调节和M1-M2表型转化,具有良好的软骨和软骨下骨修复性能。
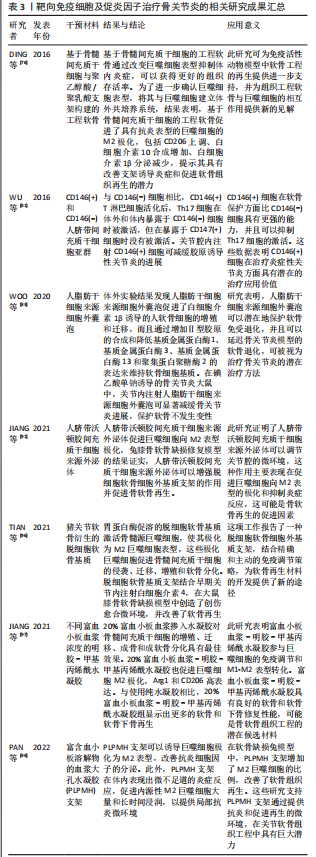
综上所述,目前尚无有效的骨关节炎治疗手段,临床主要以对症治疗、缓解症状为主,最终可能需要进行关节置换手术。随着科学的发展,干细胞及其来源细胞外囊泡可能通过多种途径调节机体免疫系统以克服骨关节炎滑膜炎症及促进软骨修复,减缓骨关节炎的进展。组织工程生物支架材料也被证实可显著减少炎性细胞及炎性因子的炎性反应,重建并修复受损部位的免疫微环境,有望成为一种更为有效、安全的骨关节炎治疗方案,见表3。
| [1] 谢辉晋.骨关节炎相关细胞因子作用机制研究进展[J].重庆医学, 2011,40(4):395-398. [2] 孙廓,张键. 细胞因子在骨关节炎中的作用研究进展[J].中国临床医学,2007,14(5):690-692. [3] 燕明岳,张永涛,张浩运,等.免疫相关因素在骨关节炎中作用的研究进展[J].中华骨科杂志,2022,42(12):791-799. [4] WANG Y, BAO M, HOU C, et al. The role of TNF-α in the pathogenesis of temporomandibular disorders. Biol Pharm Bull. 2021;44(12):1801-1809. [5] MINASHIMA T, KIRSCH T. Annexin A6 regulates catabolic events in articular chondrocytes via the modulation of NF-κB and Wnt/ß-catenin signaling. PLoS One. 2018;13(5):e0197690. [6] ZHOU RP, DAI BB, XIE YY, et al. Interleukin-1β and tumor necrosis factor-α augment acidosis-induced rat articular chondrocyte apoptosis via nuclear factor-kappaB-dependent upregulation of ASIC1a channel. Biochim Biophys Acta Mol Basis Dis. 2018;1864(1):162-177. [7] KAMIYA N, KUROYANAGI G, ARUWAJOYE O, et al. IL6 receptor blockade preserves articular cartilage and increases bone volume following ischemic osteonecrosis in immature mice. Osteoarthritis Cartilage. 2019;27(2):326-335. [8] MEHANA EE, KHAFAGA AF, EL-BLEHI SS. The role of matrix metalloproteinases in osteoarthritis pathogenesis: an updated review. Life Sci. 2019;234:116786. [9] XIE XW, WAN RZ, LIU ZP. Recent research advances in selective matrix metalloproteinase-13 inhibitors as anti-osteoarthritis agents. Chem Med Chem. 2017;12(15):1157-1168. [10] CHEN CG, THUILLIER D, CHIN EN, et al. Chondrocyte-intrinsic Smad3 represses Runx2-inducible matrix metalloproteinase 13 expression to maintain articular cartilage and prevent osteoarthritis. Arthritis Rheum. 2012;64(10):3278-3289. [11] LIM H, MIN DS, KANG Y, et al. Inhibition of matrix metalloproteinase-13 expression in IL-1β-treated articular chondrocytes by a steroidal saponin, spicatoside A, and its cellular mechanisms of action. Arch Pharm Res. 2015;38(6):1108-1116. [12] BOUMAH CE, LEE M, SELVAMURUGAN N, et al. Runx2 recruits p300 to mediate parathyroid hormone’s effects on histone acetylation and transcriptional activation of the matrix metalloproteinase-13 gene. Mol Endocrinol. 2009;23(8):1255-1263. [13] CLEMENTS KM, FLANNELLY JK, TART J, et al. Matrix metalloproteinase 17 is necessary for cartilage aggrecan degradation in an inflammatory environment. Ann Rheum Dis. 2011;70(4):683-689. [14] 闫新峰,常晓天,张明,等.ADAMTS-4在多种软骨中的表达[J].中华外科杂志,2008, 46(3):228-229. [15] MAJUMDAR MK, ASKEW R, SCHELLING S, et al. Double-knockout of ADAMTS-4 and ADAMTS-5 in mice results in physiologically normal animals and prevents the progression of osteoarthritis. Arthritis Rheum. 2007;56(11):3670-3674. [16] EGLOFF C, HART DA, HEWITT C, et al. Joint instability leads to long-term alterations to knee synovium and osteoarthritis in a rabbit model. Osteoarthritis Cartilage. 2016; 24(6):1054-1060. [17] 张文凯,党晓谦.创伤性膝骨关节炎模型兔软骨下骨的影像学结构改变[J].重庆医学,2022,51(8):1285-1289,1296. [18] 李婧瑜,苏盈盈,白丁.骨关节炎早期模型小鼠软骨下骨的形态学特征[J].中国组织工程研究,2022,26(11):1692-1698. [19] LI Y, LIEM Y, DALL’ARA E, et al. Subchondral bone microarchitecture and mineral density in human osteoarthritis and osteoporosis: a regional and compartmental analysis. J Orthop Res. 2021;39(12):2568-2580. [20] BIANCO D, TODOROV AJR, ČENGIĆ T, et al. Subchondral bone mesenchymal stromal cells from osteoarthritic lesions give rise to aberrant in vitro and in vivo mineralization. Osteoarthritis Cartilage. 2016;24:S133. [21] FINNILÄ MAJ, THEVENOT J, AHO OM, et al. Association between subchondral bone structure and osteoarthritis histopathological grade. J Orthop Res. 2017;35(4):785-792. [22] LIVSHITS G, ZHAI G, HART DJ, et al. Interleukin-6 is a significant predictor of radiographic knee osteoarthritis: the chingford study. Arthritis Rheum. 2009;60(7):2037-2045. [23] STANNUS O, JONES G, CICUTTINI F, et al. Circulating levels of IL-6 and TNF-α are associated with knee radiographic osteoarthritis and knee cartilage loss in older adults. Osteoarthritis Cartilage. 2010;18(11):1441-1447. [24] LABINSKY H, PANIPINTO PM, LY KA, et al. Multiparameter analysis identifies heterogeneity in knee osteoarthritis synovial responses. Arthritis Rheumatol. 2020;72(4):598-608. [25] 刘金富,曾平,农焦,等.整合多组微阵列芯片分析骨关节炎患者滑膜中生物标志物和治疗靶点[J].中国组织工程研究,2021,25(23):3690-3696. [26] FUTANI H, OKAYAMA A, MATSUI K, et al. Relation between interleukin-18 and PGE2 in synovial fluid of osteoarthritis: a potential therapeutic target of cartilage degradation. J Immunother. 2002;25 Suppl 1:S61-S64. [27] WANG Q, ROZELLE AL, LEPUS CM, et al. Identification of a central role for complement in osteoarthritis. Nat Med. 2011;17(12):1674-1679. [28] ASSIRELLI E, PULSATELLI L, DOLZANI P, et al. Complement expression and activation in osteoarthritis joint compartments. Front Immunol. 2020;11:535010. [29] DE LANGE-BROKAAR BJ, IOAN-FACSINAY A, VAN OSCH GJ, et al. Synovial inflammation, immune cells and their cytokines in osteoarthritis: a review. Osteoarthritis Cartilage. 2012;20(12):1484-1499. [30] UTOMO L, BASTIAANSEN-JENNISKENS YM, VERHAAR JA, et al. Cartilage inflammation and degeneration is enhanced by pro-inflammatory (M1) macrophages in vitro, but not inhibited directly by anti-inflammatory (M2) macrophages. Osteoarthritis Cartilage. 2016;24(12):2162-2170. [31] WANG F, ZHANG S, JEON R, et al. Interferon gamma induces reversible metabolic reprogramming of m1 macrophages to sustain cell viability and pro-inflammatory activity. EBioMedicine. 2018;30:303-316. [32] ZHANG H, LIN C, ZENG C, et al. Synovial macrophage M1 polarisation exacerbates experimental osteoarthritis partially through R-spondin-2. Ann Rheum Dis. 2018;77(10): 1524-1534. [33] COLLINS SL, OH MH, SUN IH, et al. mTORC1 Signaling regulates proinflammatory macrophage function and metabolism. J Immunol. 2021;207(3):913-922. [34] ZHANG H, CAI D, BAI X. Macrophages regulate the progression of osteoarthritis. Osteoarthritis Cartilage. 2020;28(5):555-561. [35] FAHY N, DE VRIES-VAN MELLE ML, LEHMANN J, et al. Human osteoarthritic synovium impacts chondrogenic differentiation of mesenchymal stem cells via macrophage polarisation state. Osteoarthritis Cartilage. 2014;22(8):1167-1175. [36] SUMOVA P, POLANSKA N, LESTINOVA T, et al. Phlebotomus perniciosus recombinant salivary proteins polarize murine macrophages toward the anti-inflammatory phenotype. Front Cell Infect Microbiol. 2020;10:427. [37] BEHRENDT P, FELDHEIM M, PREUSSE-PRANGE A, et al. Chondrogenic potential of IL-10 in mechanically injured cartilage and cellularized collagen ACI grafts. Osteoarthritis Cartilage. 2018;26(2):264-275. [38] JUNG YK, KIM GW, PARK HR, et al. Role of interleukin-10 in endochondral bone formation in mice: anabolic effect via the bone morphogenetic protein/Smad pathway. Arthritis Rheum. 2013;65(12): 3153-3164. [39] LIU F, QIU H, XUE M, et al. MSC-secreted TGF-β regulates lipopolysaccharide-stimulated macrophage M2-like polarization via the Akt/FoxO1 pathway. Stem Cell Res Ther. 2019; 10(1):345. [40] HU Y, GUI Z, ZHOU Y, et al. Quercetin alleviates rat osteoarthritis by inhibiting inflammation and apoptosis of chondrocytes, modulating synovial macrophages polarization to M2 macrophages. Free Radic Biol Med. 2019;145:146-160. [41] SESIA SB, DUHR R, MEDEIROS DA CUNHA C, et al. Anti-inflammatory/tissue repair macrophages enhance the cartilage-forming capacity of human bone marrow-derived mesenchymal stromal cells. J Cell Physiol. 2015;230(6):1258-1269. [42] DAI M, SUI B, XUE Y, et al. Cartilage repair in degenerative osteoarthritis mediated by squid type II collagen via immunomodulating activation of M2 macrophages, inhibiting apoptosis and hypertrophy of chondrocytes. Biomaterials. 2018;180:91-103. [43] DAGHESTANI HN, PIEPER CF, KRAUS VB. Soluble macrophage biomarkers indicate inflammatory phenotypes in patients with knee osteoarthritis. Arthritis Rheumatol. 2015;67(4):956-965. [44] LIU B, ZHANG M, ZHAO J, et al. Imbalance of M1/M2 macrophages is linked to severity level of knee osteoarthritis. Exp Ther Med. 2018;16(6):5009-5014. [45] MANFERDINI C, PAOLELLA F, GABUSI E, et al. From osteoarthritic synovium to synovial-derived cells characterization: synovial macrophages are key effector cells. Arthritis Res Ther. 2016;18:83. [46] SAITO I, KOSHINO T, NAKASHIMA K, et al. Increased cellular infiltrate in inflammatory synovia of osteoarthritic knees. Osteoarthritis Cartilage. 2002;10(2):156-162. [47] MADSEN DH, LEONARD D, MASEDUNSKAS A, et al. M2-like macrophages are responsible for collagen degradation through a mannose receptor-mediated pathway. J Cell Biol. 2013;202(6):951-966. [48] DAI M, LIU X, WANG N, et al. Squid type II collagen as a novel biomaterial: Isolation, characterization, immunogenicity and relieving effect on degenerative osteoarthritis via inhibiting STAT1 signaling in pro-inflammatory macrophages. Mater Sci Eng C Mater Biol Appl. 2018;89:283-294. [49] HOEKSEMA MA, GLASS CK. Nature and nurture of tissue-specific macrophage phenotypes. Atherosclerosis. 2019;281:159-167. [50] WOOD MJ, LECKENBY A, REYNOLDS G, et al. Macrophage proliferation distinguishes 2 subgroups of knee osteoarthritis patients. JCI Insight. 2019;4(2):e125325. [51] ALAHDAL M, ZHANG H, HUANG R, et al. Potential efficacy of dendritic cell immunomodulation in the treatment of osteoarthritis. Rheumatology (Oxford). 2021; 60(2):507-517. [52] LAKEY RL, MORGAN TG, ROWAN AD, et al. A novel paradigm for dendritic cells as effectors of cartilage destruction. Rheumatology (Oxford). 2009;48(5):502-507. [53] E XP, CAO Y, MENG H, et al. Dendritic cells of synovium in experimental model of osteoarthritis of rabbits. Cell Physiol Biochem. 2012;30(1):23-32. [54] MORET FM, HACK CE, VAN DER WURFF-JACOBS KM, et al. Intra-articular CD1c-expressing myeloid dendritic cells from rheumatoid arthritis patients express a unique set of T cell-attracting chemokines and spontaneously induce Th1, Th17 and Th2 cell activity. Arthritis Res Ther. 2013;15(5):R155. [55] YOSHINO H, TAKAHASHI K, MONZEN S, et al. Proteoglycans regulate the chemotaxis of dendritic cells derived from human peripheral blood monocytes. Biol Pharm Bull. 2010;33(6):938-944. [56] DUDECK A, SUENDER CA, KOSTKA SL, et al. Mast cells promote Th1 and Th17 responses by modulating dendritic cell maturation and function. Eur J Immunol. 2011;41(7):1883-1893. [57] LIN J, XIAO L, OUYANG G, et al. Total glucosides of paeony inhibits Th1/Th17 cells via decreasing dendritic cells activation in rheumatoid arthritis. Cell Immunol. 2012; 280(2):156-163. [58] FELSON DT, NIU J, NEOGI T, et al. Synovitis and the risk of knee osteoarthritis: The most study. Osteoarthritis Cartilage. 2016;24(3):458-464. [59] CRINIER A, NARNI-MANCINELLI E, UGOLINI S, et al. SnapShot: Natural Killer Cells. Cell. 2020;180(6):1280-1280.e1. [60] 彭志伟.NK细胞在膝关节骨性关节炎中的表型特征[D].合肥:安徽医科大学,2020. [61] FAN YY, YANG BY, WU CY. Phenotypically and functionally distinct subsets of natural killer cells in human PBMCs. Cell Biol Int. 2008;32(2):188-197. [62] MIKKO M, FREDRIKSSON K, WAHLSTRÖM J, et al. Human T cells stimulate fibroblast-mediated degradation of extracellular matrix in vitro. Clin Exp Immunol. 2008;151(2):317-325. [63] PETRASCA A, PHELAN JJ, ANSBORO S, et al. Targeting bioenergetics prevents CD4 T cell-mediated activation of synovial fibroblasts in rheumatoid arthritis. Rheumatology (Oxford). 2020;59(10):2816-2828. [64] LI YS, LUO W, ZHU SA, et al. T Cells in Osteoarthritis: alterations and beyond. Front Immunol. 2017;8:356. [65] PÖLLINGER B, JUNT T, METZLER B, et al. Th17 cells, not IL-17+ γδ T cells, drive arthritic bone destruction in mice and humans. J Immunol. 2011;186(4):2602-2612. [66] LI X, YUAN FL, LU WG, et al. The role of interleukin-17 in mediating joint destruction in rheumatoid arthritis. Biochem Biophys Res Commun. 2010;397(2):131-135. [67] SHUI XL, LIN W, MAO CW, et al. Blockade of IL-17 alleviated inflammation in rat arthritis and MMP-13 expression. Eur Rev Med Pharmacol Sci. 2017;21(10):2329-2337. [68] SHAHBAZ SK, SADEGHI M, KOUSHKI K, et al. Regulatory T cells: possible mediators for the anti-inflammatory action of statins. Pharmacol Res. 2019;149:104469. [69] LEI H, SCHMIDT-BLEEK K, DIENELT A, et al. Regulatory T cell-mediated anti-inflammatory effects promote successful tissue repair in both indirect and direct manners. Front Pharmacol. 2015;6:184. [70] SHEN PC, WU CL, JOU IM, et al. T helper cells promote disease progression of osteoarthritis by inducing macrophage inflammatory protein-1γ. Osteoarthritis Cartilage. 2011;19(6):728-736. [71] WU F, GAO J, KANG J, et al. B cells in rheumatoid arthritis: pathogenic mechanisms and treatment prospects. Front Immunol. 2021;12: 750753. [72] FANG Q, ZHOU C, NANDAKUMAR KS. Molecular and cellular pathways contributing to joint damage in rheumatoid arthritis. Mediators Inflamm. 2020;2020:3830212. [73] MATSUSHITA T. Regulatory and effector B cells: friends or foes? J Dermatol Sci. 2019; 93(1):2-7. [74] SUN H, ZHANG Y, SONG W, et al. IgM+CD27+ B cells possessed regulatory function and represented the main source of B cell-derived IL-10 in the synovial fluid of osteoarthritis patients. Hum Immunol. 2019;80(4):263-269. [75] RAFFAGHELLO L, BIANCHI G, BERTOLOTTO M, et al. Human mesenchymal stem cells inhibit neutrophil apoptosis: a model for neutrophil preservation in the bone marrow niche. Stem Cells. 2008;26(1):151-162. [76] DING J, CHEN B, LV T, et al. Bone marrow mesenchymal stem cell-based engineered cartilage ameliorates polyglycolic acid/polylactic acid scaffold-induced inflammation through m2 polarization of macrophages in a pig model. Stem Cells Transl Med. 2016;5(8):1079-1089. [77] LUZ-CRAWFORD P, KURTE M, BRAVO-ALEGRÍA J, et al. Mesenchymal stem cells generate a CD4+CD25+Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Res Ther. 2013;4(3):65. [78] VOSKAMP C, KOEVOET WJLM, SOMOZA RA, et al. Enhanced chondrogenic capacity of mesenchymal stem cells after TNFα pre-treatment. Front Bioeng Biotechnol. 2020; 8:658. [79] MATSUMURA E, TSUJI K, KOMORI K, et al. Pretreatment with IL-1β enhances proliferation and chondrogenic potential of synovium-derived mesenchymal stem cells. Cytotherapy. 2017;19(2):181-193. [80] FRAZIER TP, MCLACHLAN JB, GIMBLE JM, et al. Human adipose-derived stromal/stem cells induce functional CD4+CD25+FoxP3+CD127- regulatory T cells under low oxygen culture conditions. Stem Cells Dev. 2014;23(9):968-977. [81] WU CC, LIU FL, SYTWU HK, et al. CD146+ mesenchymal stem cells display greater therapeutic potential than CD146- cells for treating collagen-induced arthritis in mice. Stem Cell Res Ther. 2016;7:23. [82] 王鹏程,游洪波,毛泽楷,等.CD105+滑膜间充质干细胞的磁珠分选及成软骨能力研究[J].中华实验外科杂志,2017,34(6):921-923. [83] WOO CH, KIM HK, JUNG GY, et al. Small extracellular vesicles from human adipose-derived stem cells attenuate cartilage degeneration. J Extracell Vesicles. 2020;9(1): 1735249. [84] WANG R, JIANG W, ZHANG L, et al. Intra-articular delivery of extracellular vesicles secreted by chondrogenic progenitor cells from MRL/MpJ superhealer mice enhances articular cartilage repair in a mouse injury model. Stem Cell Res Ther. 2020;11(1):93. [85] MATHIVANAN S, JI H, SIMPSON RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010;73(10):1907-1920. [86] 凌华军,王其友,林伟文,等.骨髓间充质干细胞来源外泌体保护软骨细胞延缓骨关节炎的发生发展[J].中国组织工程研究,2021,25(31):4964-4969. [87] MENG HY, CHEN LQ, CHEN LH. The inhibition by human MSCs-derived miRNA-124a overexpression exosomes in the proliferation and migration of rheumatoid arthritis-related fibroblast-like synoviocyte cell. BMC Musculoskelet Disord. 2020;21(1):150. [88] RAGNI E, COLOMBINI A, VIGANÒ M, et al. Cartilage protective and immunomodulatory features of osteoarthritis synovial fluid-treated adipose-derived mesenchymal stem cells secreted factors and extracellular vesicles-embedded miRNAs. Cells. 2021;10(5):1072. [89] ZHANG S, CHUAH SJ, LAI RC, et al. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials. 2018;156:16-27. [90] CHEN Z, WANG H, XIA Y, et al. Therapeutic potential of Mesenchymal cell–derived miRNA-150-5p–expressing Exosomes in rheumatoid arthritis mediated by the modulation of MMP14 and VEGF. J Immunol. 2018;201(8):2472-2482. [91] JIANG S, TIAN G, YANG Z, et al. Enhancement of acellular cartilage matrix scaffold by Wharton’s jelly mesenchymal stem cell-derived exosomes to promote osteochondral regeneration. Bioact Mater. 2021;6(9):2711-2728. [92] ZHANG J, RONG Y, LUO C, et al. Bone marrow mesenchymal stem cell-derived exosomes prevent osteoarthritis by regulating synovial macrophage polarization. Aging (Albany NY). 2020;12(24):25138-25152. [93] ZHANG B, TIAN X, QU Z, et al. Hypoxia-preconditioned extracellular vesicles from mesenchymal stem cells improve cartilage repair in osteoarthritis. Membranes (Basel). 2022;12(2):225. [94] LIU W, RONG Y, WANG J, et al. Exosome-shuttled miR-216a-5p from hypoxic preconditioned mesenchymal stem cells repair traumatic spinal cord injury by shifting microglial M1/M2 polarization. J Neuroinflammation. 2020;17(1):47. [95] DA-WA ZX, JUN M, CHAO-ZHENG L, et al. Exosomes derived from M2 macrophages exert a therapeutic effect via inhibition of the PI3K/AKT/mTOR pathway in rats with knee osteoarthritic. Biomed Res Int. 2021;2021:7218067. [96] TIAN G, JIANG S, LI J, et al. Cell-free decellularized cartilage extracellular matrix scaffolds combined with interleukin 4 promote osteochondral repair through immunomodulatory macrophages: in vitro and in vivo preclinical study. Acta Biomater. 2021;127:131-145. [97] JIANG G, LI S, YU K, et al. A 3D-printed PRP-GelMA hydrogel promotes osteochondral regeneration through M2 macrophage polarization in a rabbit model. Acta Biomater. 2021;128:150-162. [98] PAN X, YUAN S, XUN X, et al. Long-term recruitment of endogenous M2 macrophages by platelet lysate-rich plasma macroporous hydrogel scaffold for articular cartilage defect repair. Adv Healthc Mater. 2022;11(6):e2101661. |
| [1] | 刘宝方, 徐 斌, 陈 雷. 葛根汤治疗骨关节炎的网络药理学分析及动物实验验证[J]. 中国组织工程研究, 2024, 28(2): 193-199. |
| [2] | 代新语, 闫纪红, 华凌军, 郑晓鸿. 抗阻运动改善超重肥胖人群身体成分:一项伞形综述[J]. 中国组织工程研究, 2024, 28(2): 267-271. |
| [3] | 龙 宜, 杨佳明, 叶 花, 钟燕彪, 王茂源. 细胞外囊泡在少肌性肥胖中的作用及机制[J]. 中国组织工程研究, 2024, 28(2): 315-320. |
| [4] | 邓 锐, 黄科铭, 罗 建 , 陈 功, 冯 健 , 黄维义, 魏 刚. 血红素氧合酶1介导阿托伐他汀在巨噬细胞极化和胆固醇蓄积中的作用[J]. 中国组织工程研究, 2024, 28(1): 62-67. |
| [5] | 龙清熙, 张萍淑, 刘 青, 欧 亚, 张丽丽, 元小冬. 单细胞RNA测序揭示星形胶质细胞的异质性[J]. 中国组织工程研究, 2024, 28(1): 139-146. |
| [6] | 农复香, 蒋志雄, 李英豪, 许文聪, 施智兰, 罗 慧, 张晴朗, 钟 爽, 唐梅文. 外泌体调控铁死亡在疾病诊断治疗中的应用与作用[J]. 中国组织工程研究, 2023, 27(在线): 1-10. |
| [7] | 申飞燕, 姚吉祥, 苏珊珊, 赵忠民, 唐巍东. 敲低环状RNA WD重复含蛋白1抑制膝骨关节炎软骨细胞增殖并诱导凋亡[J]. 中国组织工程研究, 2023, 27(在线): 1-6. |
| [8] | 李晓敏, 田向东 , 谭冶彤 , 朱光宇 , 王荣田 , 王 剑 , 薛志鹏, 马 晟, 胡元一, 黄 叶, 丁天送. 骨质疏松性内侧室膝骨关节炎胫骨高位截骨后下肢力线及膝关节功能的变化[J]. 中国组织工程研究, 2023, 27(9): 1325-1329. |
| [9] | 潘钟杰, 秦志鸿, 郑铁军, 丁晓飞, 廖世杰. 股骨头坏死发病机制中非编码RNA的靶标性[J]. 中国组织工程研究, 2023, 27(9): 1441-1447. |
| [10] | 蔡志浩, 谢召勇. 股骨颈前倾角测量评估:如何建立统一的方法和标准[J]. 中国组织工程研究, 2023, 27(9): 1448-1454. |
| [11] | 党 祎, 杜成砚, 姚红林, 袁能华, 曹 金, 熊 山, 张顶梅, 王 信. 激素型骨坏死与氧化应激[J]. 中国组织工程研究, 2023, 27(9): 1469-1476. |
| [12] | 龙桂月, 李冬冬, 廖红兵. 磷酸钙骨水泥/聚乳酸羟基乙酸降解产物促进小鼠单核细胞破骨向分化[J]. 中国组织工程研究, 2023, 27(8): 1193-1198. |
| [13] | 黄林科, 韦林华, 蒋 捷, 刘 倩, 陈蔚蔚. 雌激素与跑台运动干预卵巢切除模型小鼠骨量和关节软骨的变化[J]. 中国组织工程研究, 2023, 27(8): 1166-1171. |
| [14] | 杨芷姗, 唐正龙. Hippo信号通路中的核心因子YAP/TAZ参与骨形成的作用与机制[J]. 中国组织工程研究, 2023, 27(8): 1264-1271. |
| [15] | 王 继, 张 敏, 杨中亚, 张 龙. 体力活动干预2型糖尿病肌少症的研究现状[J]. 中国组织工程研究, 2023, 27(8): 1272-1277. |
目前治疗骨关节炎的方法均有一定的局限性,且只能部分缓解疼痛及改善关节功能,未能有效地实现关节软骨结构和生物功能的重建。而大量的科学家已经将目光放在利用细胞工程技术和组织工程技术攻克骨关节炎上。间充质干细胞在体内外特定诱导条件下可分化为成骨细胞和软骨细胞,进行骨关节软骨修复,还可分泌大量细胞因子,促进新生血管生成,显著改善关节腔内的微循环,其还具有免疫调节的生物学特性,可通过与免疫细胞交流和分泌可溶性分子的方式,调节关节腔的微环境,直接或间接调节炎性反应,是较为理想的种子细胞。由此可见,调节关节腔微环境对于软骨修复至关重要。
随着对骨关节炎研究的不断深入,各种免疫细胞和促炎细胞因子在骨关节炎发病过程中的重要性不断被阐明。以往学者大多从分解性细胞因子、合成性细胞因子、调节性细胞因子之间的平衡与否探讨与骨关节炎软骨损伤的关系[1-2],还有将重要免疫细胞及相关的免疫分子在骨关节炎发生发展中的作用进行一定的总结阐述[3],文章就免疫细胞及相关细胞因子在骨关节炎病程进展中的作用作一综述,同时,探讨了靶向免疫细胞治疗骨关节炎的研究现状,希望能对其他学者认识骨关节炎的发病机制及新的治疗方法提供帮助。 中国组织工程研究杂志出版内容重点:组织构建;骨细胞;软骨细胞;细胞培养;成纤维细胞;血管内皮细胞;骨质疏松;组织工程
1.1.1 检索人及检索时间 第一作者于2022年1月进行检索。
1.1.2 检索文献时限 检索2000年1月至2022年6月发表的相关文献。
1.1.3 检索数据库 中文数据库包括中国知网、万方及维普数据库;英文数据库包括PubMed 和Web of Science 数据库。
1.1.4 检索词 中文检索词包括“骨关节炎,巨噬细胞,T细胞,B细胞,自然杀伤细胞,树突状细胞,细胞因子,炎症因子,免疫细胞”;英文检索词包括“osteoarthritis,macrophages,T cells,B cells,natural killer cells,dendritic cells,cytokines,inflammatory factors,immune cells”。
1.1.5 检索文献类型 研究论文、综述和学位论文。
1.1.6 手工检索情况 无。
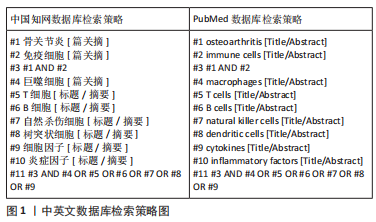
1.1.7 检索策略 以中国知网和PubMed数据库为例,检索策略图见图1。
1.2 入组标准
1.2.1 纳入标准 ①有关膝骨关节炎发病机制方面的研究;②免疫细胞对膝骨关节炎修复的影响;③基于免疫调节策略修复膝骨关节炎;④同一领域文献选择在近期发表或在权威杂志发表的文章;⑤相同领域的选择论点、论据及实验可靠的文献。
1.2.2 排除标准 ①重复性及参考价值不高的文献;②与研究目的无相关性或相关性较小的文献;③无法获取全文的文献;④发表时间过早的文献。
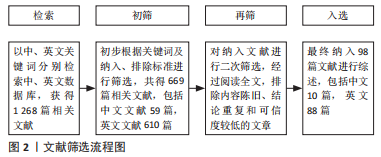
1.3 文献质量评估及数据提取 通过检索数据库获得1 268篇相关文献,按照纳入标准和排除标标准,并阅读标题和摘要进行初筛,再次阅读全文后,最后选取98篇文献进行综述,包括中文10篇,英文88篇。文献筛选流程见图2。
3.2 作者综述区别于他人他篇的特点 文章就免疫细胞及相关细胞因子在骨关节炎病程进展中的作用进行综述。免疫反应越来越被认为是影响骨关节炎修复的关键因素,遗传、代谢或机械因素导致软骨的初始损伤,软骨特异性自身抗原释放,从而激活免疫反应,包括T细胞、B细胞和巨噬细胞在内的免疫细胞浸润关节及滑膜组织,释放细胞因子和趋化因子,进一步加重损伤。同时,探讨了靶向免疫细胞治疗骨关节炎的研究现状,为创造促进软骨修复的微环境,免疫细胞已成为关键的治疗靶点,基于干细胞及其来源外泌体及生物材料进行免疫调节似乎是软骨损伤的一种潜在有效的治疗策略。
3.3 综述的局限性 骨关节炎修复需要在空间和时间上对关节炎症微环境进行多维调控,不同表型的免疫细胞在组织中发挥着截然不同的免疫功能。因此,正确调控免疫反应以及免疫微环境,有必要进一步研究区分免疫细胞亚群,确定哪些细胞可以作为修复骨关节炎的靶细胞,并研究潜在的机制。
3.4 综述的重要意义 靶向免疫细胞治疗骨关节炎的效果已在不同的实验模型中得到证实,随着新治疗靶点的发现,针对靶点进行相关治疗将为临床骨关节炎修复带来新的希望。
3.5 课题专家组对未来的建议 尽管目前的基础研究显示靶向免疫细胞治疗骨关节炎取得了不错的效果,但将其用于临床实践之前仍有大量的实验工作要做,相信未来会让骨关节炎患者从中受益。 中国组织工程研究杂志出版内容重点:组织构建;骨细胞;软骨细胞;细胞培养;成纤维细胞;血管内皮细胞;骨质疏松;组织工程
 #br#
#br#
文题释义:
骨关节炎:是一种退行性关节疾病,可逐渐导致关节功能丧失甚至身体残疾,骨关节炎最常累及的关节是膝关节,主要影响老龄人群及肥胖人群,临床症状为膝关节疼痛、肿胀、僵硬和活动能力下降,主要病理表现以软骨退变为主,进而累及滑膜、软骨下骨及关节内其他组织发生退化改变。免疫细胞:参与免疫应答或者与免疫应答有关的细胞统称为免疫细胞,包括淋巴细胞、单核吞噬细胞、巨噬细胞、自然杀伤细胞等。在免疫应答过程中,起核心作用的是淋巴细胞,其能接受抗原刺激而活化、增殖及分化。发生特异性免疫反应的淋巴细胞,称为抗原特异性淋巴细胞或免疫活性细胞,他们表面有抗原特异性受体,能特异性识别抗原。巨噬细胞和自然杀伤细胞虽然可以识别抗原,但对抗原的识别并不是特异的。
随着对骨关节炎发病机制的研究趋于深入,越来越多的数据表明免疫系统参与其中,各种免疫细胞、促炎细胞因子在骨关节炎发病过程中的重要性不断被阐明。文章就免疫细胞及相关细胞因子在骨关节炎病程进展中的作用作一综述,免疫反应越来越被认为是影响骨关节炎修复的关键因素。同时,探讨了靶向免疫细胞治疗骨关节炎的研究现状,为创造促进软骨修复的微环境,免疫细胞已成为关键的治疗靶点,基于干细胞及其来源外泌体、生物材料进行免疫调节似乎是软骨损伤的一种潜在有效的治疗策略。
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||