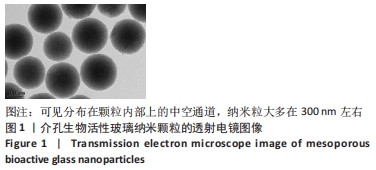
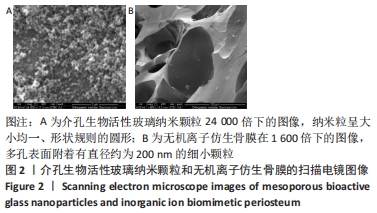
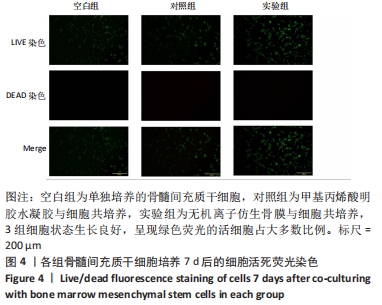
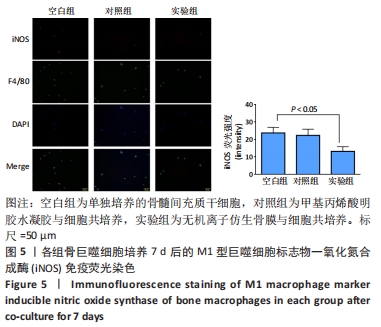
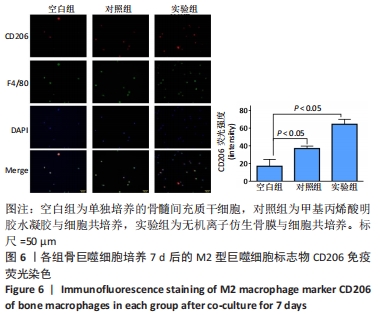
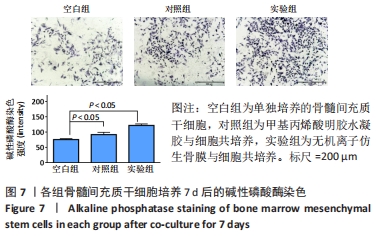
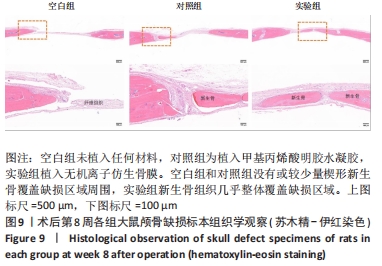
[1] 于明琨.自体骨移植修补颅骨缺损的材料与方法[J].中国临床康复, 2006,10(41):126-129.
[2] 张贝利,张伟滨,邓廉夫.同种异体骨移植研究进展[J].国际骨科学杂志,2005,26(3):164-167.
[3] BAO X, ZHU L, HUANG X, et al. 3D biomimetic artificial bone scaffolds with dual-cytokines spatiotemporal delivery for large weight-bearing bone defect repair. Sci Rep. 2017;7(1):1-13.
[4] AUGUSTIN G, ANTABAK A, DAVILA S. The periosteum. Part 1: Anatomy, histology and molecular biology. Injury. 2007;38(10):1115-1130.
[5] FILLINGHAM Y, JACOBS J. Bone grafts and their substitutes. Bone Joint J. 2016;98-b(1 Suppl A):6-9.
[6] LI N, SONG J, ZHU G, et al. Periosteum tissue engineering-a review. Biomater Sci. 2016;4(11):1554-1561.
[7] KOH TJ, DIPIETRO LA. Inflammation and wound healing: the role of the macrophage. Expert Rev Mol Med. 2011;13:e23.
[8] MANTOVANI A, BISWAS SK, GALDIERO MR, et al. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. 2013;229(2): 176-185.
[9] LEE J, BYUN H, MADHURAKKAT PERIKAMANA SK, et al. Current Advances in Immunomodulatory Biomaterials for Bone Regeneration. Adv Healthc Mater. 2019;8(4):e1801106.
[10] MOSSER DM, EDWARDS JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958-969.
[11] JIN SS, HE DQ, LUO D, et al. A Biomimetic Hierarchical Nanointerface Orchestrates Macrophage Polarization and Mesenchymal Stem Cell Recruitment To Promote Endogenous Bone Regeneration. ACS Nano. 2019;13(6):6581-6595.
[12] JI X, YUAN X, MA L, et al. Mesenchymal stem cell-loaded thermosensitive hydroxypropyl chitin hydrogel combined with a three-dimensional-printed poly(ε-caprolactone) /nano-hydroxyapatite scaffold to repair bone defects via osteogenesis, angiogenesis and immunomodulation. Theranostics. 2020;10(2):725-740.
[13] LV L, XIE Y, LI K, et al. Unveiling the mechanism of surface hydrophilicity-modulated MAcrophage polarization. Adv Healthc MAter. 2018;7(19): e1800675.
[14] QIAN Y, LI L, SONG Y, et al. Surface modification of nanofibrous matrices via layer-by-layer functionalized silk assembly for mitigating the foreign body reaction. Biomaterials. 2018;164:22-37.
[15] CHEN Z, MAO X, TAN L, et al. Osteoimmunomodulatory properties of magnesium scaffolds coated with β-tricalcium phosphate. Biomaterials. 2014;35(30):8553-8565.
[16] XIN T, MAO J, LIU L, et al. Programmed Sustained Release of Recombinant Human Bone Morphogenetic Protein-2 and Inorganic Ion Composite Hydrogel as Artificial Periosteum. ACS Appl Mater Interfaces. 2020;12(6):6840-6851.
[17] GU Q, YANG H, SHI Q. Macrophages and bone inflammation. J Orthop Translat. 2017;10:86-93.
[18] XIN T, GU Y, CHENG R, et al. Inorganic Strengthened Hydrogel Membrane as Regenerative Periosteum. ACS Appl Mater Interfaces. 2017;9(47):41168-41180.
[19] WU L, GU Y, LIU L, et al. Hierarchical micro/nanofibrous membranes of sustained releasing VEGF for periosteal regeneration. Biomaterials. 2020;227:119555.
[20] Tang J, Gu Y, ZHANG H, et al.Outer-inner dual reinforced micro/nano hierarchical scaffolds for promoting osteogenesis. Nanoscale 2019;11(34):15794-15803.
[21] MILLS CD, THOMAS AC, LENZ LL, et al. Macrophage: SHIP of Immunity. Front Immunol. 2014;5:620.
[22] GRANEY PL, ROOHANI-ESFAHANI SI, ZREIQAT H, et al. In vitro response of macrophages to ceramic scaffolds used for bone regeneration. J R Soc Interface. 2016;13(120):20160346.
[23] GONG L, ZHAO Y, ZHANG Y, et al. The MAcrophage polarization regulates MSC osteoblast differentiation in vitro. Ann Clin Lab Sci. 2016;46:65e71.
[24] HUANG Y, WU C, ZHANG X, et al. Regulation of immune response by bioactive ions released from silicate bioceramics for bone regeneration. Acta Biomater. 2018;66:81-92.
[25] LI T, PENG M, YANG Z, et al. 3D-printed IFN-γ-loading calcium silicate-β-tricalcium phosphate scaffold sequentially activates M1 and M2 polarization of macrophages to promote vascularization of tissue engineering bone. Acta Biomater. 2018;71:96-107.
[26] 武敬文,杨岚,李源静,等.介孔生物活性玻璃研究进展[J].生物医学工程学杂志,2018,35(4):153-156.
[27] HENCH LL, SPLINTER RJ, ALLEN WC, et al. Bonding mechanisms at the interface of ceramic prosthetic materials. J Biomed Mater Res. 1971;5(6):117-141.
[28] CHEN C, TANG J, GU Y, et al. Bioinspired Hydrogel Electrospun Fibers for Spinal Cord Regeneration. Adv Functl Mater. 2019;29(4). doi.org/10.1002/adfm.201806899
[29] ZHU D, SU Y, YOUNG ML, et al. Biological Responses and Mechanisms of Human Bone Marrow Mesenchymal Stem Cells to Zn and Mg Biomaterials. ACS Appl Mater Interfaces. 2017;9(33):27453-27461.
[30] XI K, GU Y, TANG J, et al. Microenvironment-responsive immunoregulatory electrospun fibers for promoting nerve function recovery [published correction appears in Nat Commun. Nat Commun. 2020;11(1):4504.
[31] WU T, CHENG N, XU C, et al. The effect of mesoporous bioglass on osteogenesis and adipogenesis of osteoporotic BMSCs. J Biomed MAter Res A. 2016;104(12):3004-3014.
[32] MIRON RJ, SCULEAN A, SHUANG Y, et al. Osteoinductive potential of a novel biphasic calcium phosphate bone graft in comparison with autographs, xenografts, and DFDBA. Clin Oral Implants Res. 2016; 27(6):668-675.
[33] BOSCH C, MELSEN B, VARGERVIK K. Importance of the critical-size bone defect in testing bone-regenerating materials. J Craniofac Surg. 1998;9(4):310-316.
[34] PAJARINEN J, LIN T, GIBON E, et al. Mesenchymal stem cell-macrophage crosstalk and bone healing. Biomaterials. 2019;196:80-89.
[35] GU Q, YANG H, SHI Q. Macrophages and bone inflammation. J Orthop Translat. 2017;10:86-93.
[36] VASCONCELOS DP, FONSECA AC, COSTA M, et al. Macrophage polarization following chitosan implantation. Biomaterials. 2013; 34(38):9952-9959
|