中国组织工程研究 ›› 2022, Vol. 26 ›› Issue (32): 5119-5125.doi: 10.12307/2022.847
• 组织构建实验造模 experimental modeling in tissue construction • 上一篇 下一篇
沉默信息调节因子6基因敲除模型小鼠肠黏膜形态及转录组学的特征性变化
游弋晖1,宋春晖1,陈 曦1,姚晓宏1,柯俊羽1,杜 群1,宋 宁2,李燕舞1
- 1广州中医药大学科技创新中心,脾胃研究所,广东省广州市 510405;2河南中医药大学药学院,河南省郑州市 450046
Characteristic changes of intestinal mucosal morphology and transcriptomics in silent information regulator 6 gene knockout mice
You Yihui1, Song Chunhui1, Chen Xi1, Yao Xiaohong1, Ke Junyu1, Du Qun1, Song Ning2, Li Yanwu1
- 1Science and Technology Innovation Center, Institute of Spleen and Stomach, Guangzhou University of Chinese Medicine, Guangzhou 510405, Guangdong Province, China; 2School of Pharmacy, Henan University of Chinese Medicine, Zhengzhou 450046, Henan Province, China
摘要:
文题释义:
肠类器官:来源于隐窝干细胞,在体外3D培养技术下,通过隐窝干细胞可增殖分化成各种功能的肠上皮细胞,可在体外重建出类似体内肠道细胞组成、生理结构和功能的三维立体肠组织。
沉默信息调节因子(Silent information regulator,SIRT):是具有去乙酰化酶活性的第三类去乙酰化酶的总称,主要通过依赖烟酰胺腺嘌呤二核苷酸发挥去乙酰化作用,SIRT家族参与了细胞新陈代谢、DNA损伤修复等细胞生命过程。
SIRT6:是SIRT家族的成员之一,具有去乙酰化酶活性和单ADP核糖基转移酶活性,在调控衰老、糖酵解、脂质代谢、炎症、肿瘤和凋亡的发生发展中发挥着不可忽视的作用。
背景:肠黏膜更新是维持机体稳态的关键生理过程,其中隐窝干细胞是肠上皮增殖和分化的驱动力。沉默信息调节因子(silent information regulator,SIRT)参与细胞的基因修复、代谢、能量平衡和寿命的调节,在肠上皮稳态中的研究逐步成为热点。肠类器官由单个隐窝干细胞经3D培养形成,可体外呈现肠上皮更新及受损后修复过程,是研究肠黏膜更新的最新模式。
目的:探究SIRT6基因特异性敲除(SIRT6-i/-i)小鼠模型肠黏膜形态、肠类器官形成及其转录组学的特征性变化。
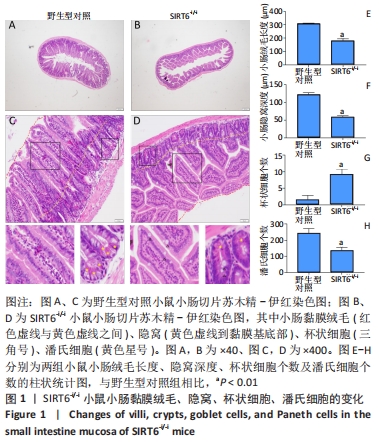
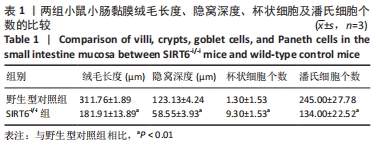
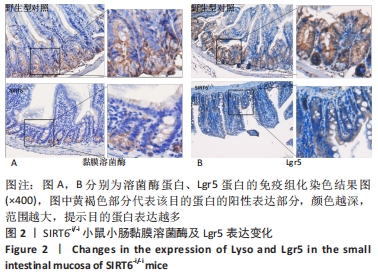
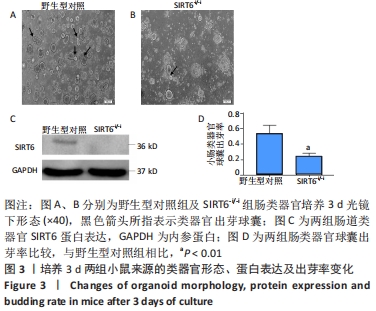
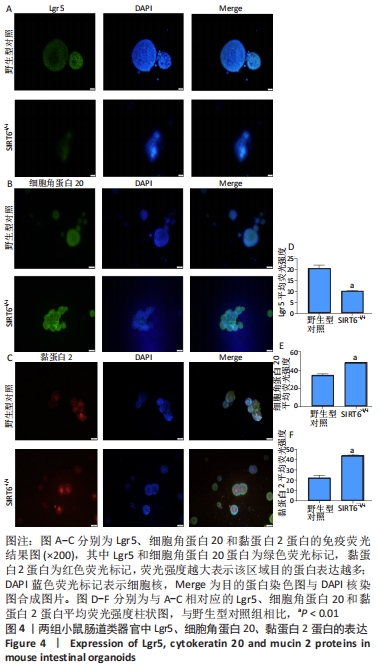
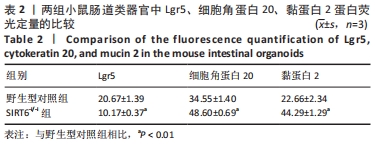
方法:①采用Cre-loxp方法建立SIRT6-i/-i小鼠模型,制备小肠组织石蜡切片并进行苏木精-伊红染色,观察其病理变化,免疫组化检测肠干细胞标记物Lgr5和潘氏细胞标记物溶菌酶的表达;②体外构建SIRT6-i/-i小鼠肠类器官模型,Western-blot检测类器官SIRT6蛋白变化,免疫荧光检测肠类器官Lgr5、细胞角蛋白20和黏蛋白2蛋白表达变化;③采用肠组织进行转录组测序,分析该模型小鼠差异基因及相关功能的特征变化。
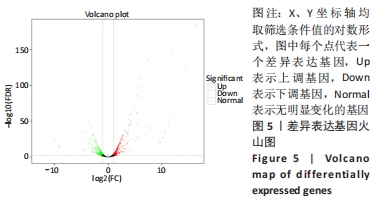
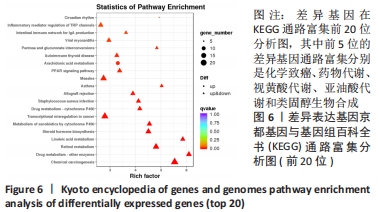
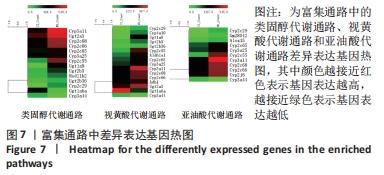
结果与结论:①与野生型对照小鼠肠黏膜形态比较,SIRT6-i/-i小鼠肠黏膜出现绒毛稀疏、变短,隐窝高度变浅,且杯状细胞数目增多,潘氏细胞数目明显减少,Lgr5和溶菌酶的表达明显降低;②SIRT6-i/-i小鼠肠类器官中SIRT6蛋白表达明显降低,培养中出芽率明显减少,且Lgr5表达降低,吸收上皮细胞标记物细胞角蛋白20和杯状细胞标记物黏蛋白2表达水平升高;③转录组结果显示,与野生型对照小鼠比较,模型小鼠肠组织的差异基因有846个,其中上调基因438个,下调基因408个,差异基因在京都基因与基因组百科全书通路富集前5位的分别是化学致癌、药物代谢、视黄酸代谢、亚油酸代谢和类固醇生物合成,显著富集通路中关联的基因主要为Cyp2c29、Cyp2c65、Cyp3a11、Cyp3a25等Cyp450家族的改变;④结果表明,SIRT6缺失会导致肠道干细胞增殖和分化功能失衡,其机制可能与SIRT6调控代谢通路有关,其中SIRT6对细胞色素P450家族的调控与肠黏膜更新稳态关系密切。
缩略语:沉默信息调节因子6:silent information regulator 6,SIRT6;小肠绒毛SIRT6基因特异性敲除小鼠:small intestinal villi SIRT6 gene-specific knockout mice,SIRT6-i/-i;京都基因与基因组百科全书:Kyoto encyclopedia of genes and genomes,KEGG
https://orcid.org/0000-0003-4032-8222 (游弋晖)
中国组织工程研究杂志出版内容重点:组织构建;骨细胞;软骨细胞;细胞培养;成纤维细胞;血管内皮细胞;骨质疏松;组织工程
中图分类号: