中国组织工程研究 ›› 2022, Vol. 26 ›› Issue (25): 4034-4045.doi: 10.12307/2022.411
• 干细胞综述 stem cell review • 上一篇 下一篇
细胞外基质力学微环境与细胞间相互作用的机制与特征
闵子洋,穆妮热·艾力,郑耘昊,曾幸芷,边楠雁,邓双珊,谢 静
- 四川大学华西口腔医学院,口腔疾病研究国家重点实验室,四川省成都市 610041
-
收稿日期:2020-11-20接受日期:2021-03-06出版日期:2022-09-08发布日期:2022-01-26 -
通讯作者:谢静,教授,博士生导师,四川大学华西口腔医学院,口腔疾病研究国家重点实验室,四川省成都市 610041 -
作者简介:闵子洋,男,2000年生,云南省玉溪市人,汉族,四川大学华西口腔医学院本科生在读,现随谢静教授进行基础医学(主要是组织工程支架力学性质方面)方向的学习研究。 -
基金资助:国家自然科学基金(81600840,81771047),项目负责人:谢静
Mechanism and characteristics of mechanical microenvironment of extracellular matrix and intercellular interaction
Min Ziyang, Munire·Aili, Zheng Yunhao, Zeng Xingzhi, Bian Nanyan, Deng Shuangshan, Xie Jing
- State Key Laboratory of Oral Diseases, West China School of Stomatology, Sichuan University, Chengdu 610041, Sichuan Province, China
-
Received:2020-11-20Accepted:2021-03-06Online:2022-09-08Published:2022-01-26 -
Contact:Xie Jing, Professor, Doctoral supervisor, State Key Laboratory of Oral Diseases, West China School of Stomatology, Sichuan University, Chengdu 610041, Sichuan Province, China -
About author:Min Ziyang, State Key Laboratory of Oral Diseases, West China School of Stomatology, Sichuan University, Chengdu 610041, Sichuan Province, China -
Supported by:the National Natural Science Foundation of China, No. 81600840, 81771047 (to XJ)
摘要:
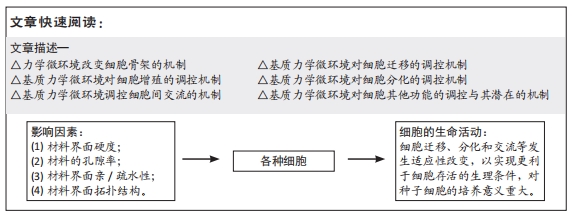
文题释义:
细胞外基质力学微环境:组织工程进行细胞培养时,支架材料为细胞提供的生存环境称细胞外基质微环境,而此处的“力学”强调了材料界面的硬度、孔隙率、拓扑结构和亲/疏水等力学性质。
背景:细胞外基质力学微环境通过调控细胞黏附、迁移、增殖和分化等,在人体器官发育、生理功能维持和疾病发生等方面发挥了重要作用。在再生医学和干细胞疗法中,基质力学微环境能引导植入细胞的存活、生长、增殖与分化,对调控植入细胞的行为发挥了重要的作用,对组织再生和干细胞治疗的成功率也起到关键作用。
目的:综述细胞外基质微环境的力学信号对细胞行为包括细胞骨架重建、迁移、增殖、分化和细胞间交流的影响,从中阐明现有的分子调控机制,以期为组织工程和干细胞疗法中细胞与其力学微环境相互作用的实践性转化提供理论支撑。
方法:检索PubMed数据库、万方数据库、CNKI中国期刊全文数据库收录的相关文献。英文检索词为“cytoskeleton,cell spreading,cell migration,cell proliferation,cell differentiation,cell communication,mechanotransduction,stiffness of substrate,surface topography,extracellular matrix,matrix”,中文检索词为“细胞骨架,细胞扩展,细胞迁移,细胞增殖,细胞分化,细胞交流,机械传导,基质硬度,表面形貌,细胞外基质,基质”,最终纳入161篇文献进行归纳总结。
结果与结论:细胞外基质各种力学信号,如材料界面硬度、拓扑结构和亲/疏水性等,从力学识别、力学-化学信号转导、信号通路级联、下游蛋白活化、转录启动到蛋白表达来调控细胞的扩展、增殖、迁移、分化、交流和其他各种特殊生理性质,这对组织工程中细胞的定向培养和临床医学中的细胞靶向治疗具有重要意义。
https://orcid.org/0000-0001-8156-0322 (谢静)
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
中图分类号:
引用本文
闵子洋, 穆妮热·艾力, 郑耘昊, 曾幸芷, 边楠雁, 邓双珊, 谢 静. 细胞外基质力学微环境与细胞间相互作用的机制与特征[J]. 中国组织工程研究, 2022, 26(25): 4034-4045.
Min Ziyang, Munire·Aili, Zheng Yunhao, Zeng Xingzhi, Bian Nanyan, Deng Shuangshan, Xie Jing. Mechanism and characteristics of mechanical microenvironment of extracellular matrix and intercellular interaction[J]. Chinese Journal of Tissue Engineering Research, 2022, 26(25): 4034-4045.
目前已有许多文献专门报道了材料界面硬度、粗糙度和基质种类等对成体细胞骨架的影响,在此将结合组织工程技术和临床需求,对材料界面-细胞骨架的交互作用进行回顾性阐述。关节软骨损伤是骨科较为常见的一种疾病,由于软骨组织无血液供应,营养来源于周围关节滑液的滋润,损伤后很难再生。传统的软骨损伤治疗方法不少,但各自均有明显劣势,如机械术式难以将损伤组织修复至正常;自体组织移植虽疗效较好,但供材有限;异体材料来源相对较多,但可能引起免疫排斥反应,且有传播疾病的危险。在此现状下,利用组织工程方法研制受损伤组织替代品是极有潜力的治疗方法。因此,探究清楚生物支架对细胞生理活动的意义重大[7-8]。目前,ZHANG等[9]已报道,软骨细胞内存在细胞骨架马达蛋白(myosin ⅡA)、G蛋白酶RhoA (Ras homolog gene family,member A)及其下游靶效应分子RhoA激酶ROCK (Rho Kinase),它们在细胞骨架重组方面有重要作用;SCHUH等[10]最近的一项研究也表明,将软骨细胞在二维基质表面培养或封装在三维水凝胶中,细胞处在坚硬材料表面时能更好地保持其形态。在心脏相关疾病方面,冠状动脉粥样硬化性心脏病已成为较常见的心脏病之一,也是世界上主要致死疾病之一。目前针对局部缺血性心脏病的干预疗法主要为药物干预和手术治疗,然而因心肌的再生修复能力极弱,这些方法均无法弥补已缺失的心肌组织,因此,利用组织工程培养心肌细胞并植入患者心肌缺损处成为极有潜力的一种潜在策略[11]。虽然在体内和体外研究中都观察到细胞疗法有助于改善心脏功能,但许多挑战仍阻碍着这项技术的应用,如较低的细胞存活率和长期安全问题等。因此,掌握材料界面的力学性质与心肌细胞的交互作用显得至关重要。JACOT等[12-16]研究表明,材料界面硬度对大鼠心肌细胞的排列、伸长和收缩性能都有明显的影响和改善作用;相较于更硬或更软的基质,细胞在硬度更接近心肌的凝胶(即中等硬度)上能产生更大的机械应力,实现更好的形态变化以适应所处微环境。XIE等[17]研究还表明,来亨鸡胚心肌细胞可感知基底硬度变化,并通过黏着斑向胞内转导信号,调整肌动蛋白的装束与胞外应力纤维的组构,使得更多的Myosin ⅡA得以产生,Myosin ⅡA进而在胞质内激活RhoA通路,使其下游蛋白ROCK触发基因的转录从而最终使细胞骨架肌动蛋白重塑,当这些蛋白表达产生的应力与外界持平时,细胞形态保持稳定。人体研究中也有类似的报道,FORTE等[18]表明,基底硬度会影响新生儿心肌细胞的形态。此外,在原代人脐静脉内皮细胞和永生化的人微血管内皮细胞中也有相应的实验证明,这两类细胞都通过在较硬基质上增加牵引力与压力来响应内皮下硬度的变化,并表现出一系列与力学信号转导有关的蛋白磷酸化或蛋白质构象变化[19]。然而也有与之前神经元相关研究矛盾的实验结果出现,KAYAL等[20]发现在凝胶上硬度梯度不同部位的神经元突起长度方面无显著差异。归因于实验材料的选取和实验设计等因素,实验结果也不尽相同,对成体细胞的研究有待进一步深入和完善。
众所周知,干细胞作为理想的组织工程种子细胞,具有临床应用前景,而在干细胞研究中,因受到疏水性、材料硬度和材料孔径的影响,主要呈现出形态学的改变。在疏水性研究方面,干细胞已被证明直接受疏水性的影响而改变其形态与扩展(其他如Hela细胞和MC3T3-E1类成骨细胞中也有类似的结论)[21-23]。在材料硬度方面,基质硬度调节的细胞骨架改变也已在多种干细胞中被证实,如骨髓间充质干细胞、诱导多功能干细胞和肾祖细胞等[24-28],且其在调控干细胞骨架的机制上与成体细胞类似。此外,胞质内细胞骨架和核内核纤层纤维蛋白共同维持了细胞核的大小、长宽比等形态学特征,SUN等[29]研究表明,人脂肪干细胞的细胞核大小和长宽比同样受到材料界面——静电纺丝硬度的影响。由此可见,基质硬度不仅影响细胞的形态,更深层次地影响着细胞控制中心——细胞核的表征。在材料孔径方面,BRENNAN等[30]则表明,将人骨髓间充质干细胞种植于熔融电纺技术模拟细胞外基质的3D纤维结构中,较小的即100 μm左右孔径大小下培养的干细胞表现出最高的种植效率和铺展的细胞形态,同时还显著增强了胶原蛋白和矿物质的沉积。
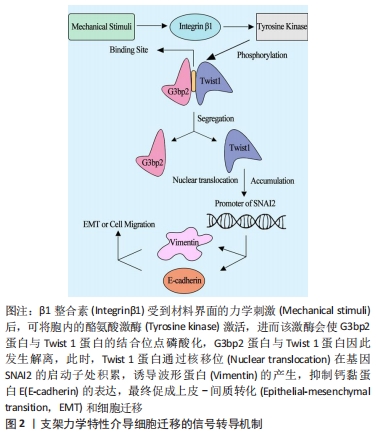
2.2 基质力学微环境对细胞迁移的调控机制 细胞与基质的连接和细胞运动相关结构的形成是决定细胞迁移的两大要素,当材料界面的力学信号传递至质膜上的介导蛋白后,通过信号通路如ROCK-MLC2的转导,其下游蛋白调控转录相关因子与核内特定基因的启动子区结合,调节与细胞-基质连接相关蛋白的表达或细胞运动结构如伪足的形成,最终实现细胞响应材料界面变化的迁移行为。与细胞的形态和扩展相类似,微管和微丝在细胞迁移中起到极为重要的作用。细胞运动依赖于微管的马达分子:驱动蛋白、动力蛋白,该过程需ATP提供能量,同时微管参与鞭毛、纤毛构成,也参与鞭毛、纤毛等细胞器结构的运动;与微管不同,微丝在细胞中可成网、成束或纤维状分散分布,它参与构成微绒毛、运动细胞的伪足,此外,它也与细胞皮层凝胶状态和溶胶状态的相互转换以及其参与的细胞多种运动如阿米巴运动、吞噬、胞质环流等有密切关系。与细胞骨架的重构相类似,当力学信号传导至细胞膜,信号通过膜蛋白的转导进入胞内,产生一系列级联反应,进而调控细胞核内特定基因的转录程度,从而调节相关蛋白的表达以调节马达分子和运动结构的形成。此外,细胞与基质的相互作用,如黏着连接中的黏着斑、半桥粒连接,还有细胞针对基质产生的金属蛋白酶,都对细胞迁移产生重要的影响,细胞与基质连接的多少,决定着细胞迁移的难易程度,而金属蛋白酶则帮助细胞分解或重新组构细胞外基质,进而完成细胞迁移。受硬度调控的细胞迁移的完整分子机制-Twist 1/G3bp2信号通路已被发现[31]。碱性螺旋-环-螺旋转录因子Twist 1是一种在肿瘤中诱导上皮-间质转化,促进细胞迁移、侵袭的蛋白[32]。该蛋白通过与固定在细胞质的Ras-GTP酶激活蛋白SH3域结合蛋白2(Ras-GTPase activating protein SH3 domain-binding protein 2,G3bp2)的特异性序列结合而定位于细胞质。当基质硬度较高的力学信号传递至质膜外表面的β1整合素时,酪氨酸激酶被激活,从而将Twist 1和G3bp2结合序列中的Tyr107磷酸化,导致Twist 1和G3bp2的解聚,Twist 1进一步移位至细胞核并积累,促进锌指转录因子Slug抗体SNAI2的转录,最终抑制了钙黏蛋白E的表达,诱导了波形蛋白的产生,促使上皮-间质转化、细胞迁移和侵袭,见图2。
对于成体细胞而言,材料界面的孔隙大小和粗糙度均能影响其迁移,但最主要的影响力学信号还是材料硬度。在材料孔隙方面,MIRON-MENDOZA等[33-34]发现材料基质的孔隙率和孔隙大小均能对细胞的运动活性产生差异性调节,其中孔隙大小是通过调控细胞间的黏着连接面积来影响细胞迁移的。在材料粗糙度方面,PHAM等[35]发现聚二甲基硅氧烷材料基质表面的形貌会影响细胞的迁移效率。在材料硬度方面,人体四大组织中的3种都已有相应细胞受硬度影响的现象被证实:①上皮组织细胞:细胞外基质的硬度刺激甚至会对上皮细胞造成深远的影响,过去的基质刚度对上皮细胞集体迁移有一种先前未知的调节,其中这种“力学记忆”依赖于YAP蛋白的活性[36]。②结缔组织细胞:细胞迁移的信号分子级联、运动形式乃至运动的具体行为都已被揭示,其中T细胞进入炎症组织的过程涉及T细胞内皮屏障牢固黏附的脱离、扩散和迁移。有研究发现该过程需要结合蛋白(CT10 regulator of kinase,Crk)的参与,因为Crk能辅助T细胞感知响应底物硬度,此外Crk蛋白还是LFA-1信号通路和肌动蛋白重塑的关键中间体,对T细胞的迁移具有重要作用[37]。细胞的运动形式在不同细胞内各不相同,培养在较软基质上的软骨细胞边缘出现了板状伪足结构,伪足是细胞运动能力强的典型特征,通过伪足在向前运动中发挥启动和推动的作用[38];而成骨细胞的迁移则体现出两面性,与软骨细胞类似,在较软材料界面上成骨细胞长出了突出丝足和伪足,然而在较硬材料界面上成骨细胞则通过基质金属蛋白酶降解基质来实现迁移,不同运动形式的转变在细胞响应材料界面的过程中极具参考价值[39-40]。迁移的具体行为也在中性粒细胞中得以揭示,多种整合素的存在可能是细胞对底物硬度的一种适应机制,中性粒细胞可以根据接收到的材料界面力学信号的梯度差异执行反转、掉头或锁定等行为来实现对材料界面硬度的应答[41]。成纤维细胞的趋硬性也在KUO等[42]研究中得到证实。③神经组织细胞:有文献报道称,胶质细胞于软胶原凝胶上的迁移在一定范围内受到抑制;与成骨细胞相似,在软胶原凝胶上的胶质细胞中很难观察到丝状伪足,但在硬胶原凝胶上则有伪足的产生且细胞比在软胶原凝胶上分布更广,由此可见,胶质细胞的迁移依赖于基质硬度[43],且迁移速度的两相变化还受到基质维度的影响[44]。
相较于成体细胞而言,病理状态下的细胞常常涉及其转移与扩散,因此,有关病理细胞迁移受材料力学信号影响的研究较为详尽,可大致分为受材料通道或孔隙影响和受材料界面硬度影响两方面。①受材料通道或孔隙的影响:材料界面如何影响癌细胞的迁移,在临床上极富指导意义。癌细胞的迁移、放疗和受损组织的再生都与此密切相关。凝胶的力学性质如宽度、孔隙大小、黏附程度、三维结构等对癌细胞和急性早幼粒细胞白血病细胞的表型、迁移具有重要影响,其中细胞的孔隙大小和低黏附性都是使细胞高效迁移的主要前提[45-48]。②受材料界面硬度的影响:癌细胞的迁移与信号通路RhoA/ROCK-MLC2/Smurf1密切相关,此通路可调控板状伪足的形成进而诱导癌细胞的迁移。材料硬度对癌细胞迁移的影响主要来自两方面,一是通过肌球蛋白Ⅱ-FAK/Cas途径促进了侵袭性伪足的激活和形成,二是影响癌细胞与基质形成的黏着斑结构,降低其间的黏附作用,以便于癌细胞更高效地迁移。从基底膜穿透、结缔组织侵袭再到基质微环境和跨屏障迁移,癌细胞间和癌细胞与细胞外基质间的交互作用(包括力学信号)至关重要,对此过程的探究有利于研究人员深层次把握癌细胞迁移的机制[49-51]。
关于人体生理状态下干细胞迁移受材料力学性质影响的研究还鲜有报道,目前主要集中于以下几个方面。Lin等[52-54]采用不同类型的凝胶模拟干细胞的三维生长环境,相较于软材料界面上的细胞而言,在硬界面上培养的骨髓间充质干细胞和神经祖细胞具有更强的迁移活动性,其中又以间充质干细胞的的信号分子机制研究最为透彻,波形蛋白通过整合来自物理微环境的力学信号输入来调节微管和肌动球蛋白网络的组装,进而促进间充质干细胞的迁移。
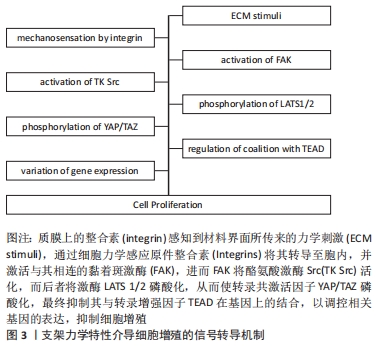
2.3 基质力学微环境对细胞增殖的调控机制 细胞增殖受细胞所处微环境物理性质的调控与细胞迁移相类似,增殖也是通过质膜上蛋白介导、胞内蛋白转导、特定基因表达改变等一系列途径来实现的,目前较清晰的是YAP信号机制[55-56]。受体超家族成员6是由几层不同蛋白质组成的大型动态多蛋白复合体,包含整合素、纽蛋白、踝蛋白和黏着斑激酶,其中黏着斑可感受到细胞外基质硬度的增加,特别是通过β1整合素传递此力学信号,激活黏着斑激酶,进而激活酪氨酸激酶Src。酪氨酸激酶Src随后将激酶LATS 1/2磷酸化,活化后的激酶LATS 1/2又可将YAP蛋白与转录共激活因子TAZ磷酸化,并将其固定于细胞骨架进而抑制这两种蛋白的活性,而只有未磷酸化的YAP会结合转录增强因子TEAD并核移位至DNA上与细胞增殖相关的基因处,促进其转录,介导细胞的增殖,见图3。对于成体细胞而言,外力作用、材料孔隙、材料表面粗糙度和材料硬度均会影响细胞增殖,而有关材料硬度的报道更为丰富。在外力作用方面,力学刺激负压(-16.7 kPa)作用12 h可诱导成骨细胞的增殖[57]。在材料孔隙方面,有研究显示微孔退火颗粒的颗粒大小和材料孔隙均会影响成纤维细胞的增殖,较大的颗粒有利于细胞增殖[58]。MITTAL等[59]发现,随有孔聚乳酸微粒支架的孔密度增加,人真皮成纤维细胞的增殖能力会降低。此外,也有报道称,在纳米尺度小至13 nm 的表面纳米孔上培养可促进纤维母细胞的增殖。
在表面粗糙度方面,有文献表明表面粗糙度会影响细胞增殖[38]。KUNZLER等[60]也发现,人牙龈成纤维细胞的增殖能力随着基质表面粗糙度的上调而减弱。在材料硬度方面,研究对象可大致分为生理状态和病理状态下的细胞。①生理状态的细胞:GU等[61]通过检测特定细胞内增殖标记蛋白在不同硬度基质上的表达,来证实基质硬度对细胞增殖的影响,其发现在硬度为0.53 kPa的支架材料上培养的许旺细胞最大增殖率远远低于先前报道的最佳硬度,这可能是由于2D和3D环境的差异造成的。实验结果表明,细胞在不同硬度基质上培养后,其增殖能力发生了变化,培养48 h后,在硬度为13-16 kPa,35-38 kPa,48-53 kPa和62-68 kPa的4组基质中S期细胞百分比分别为26.82%,26.64%,24.43%和22.39%;培养72 h后,上述硬度下的4组基质中S期细胞百分比分别为16.30%,16.12%,15.68%和10.88%。由此可见,许旺细胞的增殖深受基质硬度的影响。在上皮细胞中,也有研究表示不同硬度基质上的角膜上皮细胞内增殖标记蛋白的含量不同[62];还有研究发现,基质硬度的上升会促进细胞外信号调节激酶磷酸化,导致内皮细胞增殖能力上升[63]。在免疫细胞中,有研究指出随着B细胞受体信号的增强和黏附分子的增多,B细胞的力学信号传感可有效调节B细胞的激活、增殖和随后的抗体反应[64]。在软骨细胞中,交联壳聚糖硬度的增加似乎也作为一种额外的力学刺激来促进软骨细胞的增殖[65]。还有文献称,较硬基质上的FAK-p130Cas-RAC信号通路激活诱导了N-钙黏素的活化,N-钙黏素促使平滑肌细胞对血管损伤进行增殖反应应答[66]。此外,贺小涛等[67]发现,高硬度基质胶复合白细胞介素4/基质细胞衍生因子1可促进牙周组织的内源性再生。②病理状态的细胞:有文献证明,在药物与基质刚度的共同作用下,相同浓度的顺铂药物中,人宫颈癌 HeLa 细胞的存活率随基底刚度的增加而下降[68]。还有研究表示,肝癌细胞所处力学微环境对肝癌细胞的增殖有重要影响;较大的基质刚度可能通过调控糖代谢途径促进肝癌细胞的增殖[69]。还有研究发现,基质刚度和固体应力对癌间质细胞的增殖和转移潜能有影响[70]。
在干细胞层面的研究主要集中于材料硬度的影响。有关材料硬度的报道中,ARSHI等[71]研究表明,基质硬度不仅是使已分化的心肌细胞成熟的独立因素,而且是诱导未分化的祖细胞向心肌细胞增殖的重要因素;CHITTETI等[72]表明,无论三维胶原低聚物基质培养体系中是否存在成骨细胞,造血祖细胞的增殖减弱和克隆形成能力的增强都与基质硬度的增加有关。
FIORETTA等[73]研究表明,在软、中、硬3个层级的聚丙烯酰胺凝胶上进行培养时,内皮集落形成细胞的黏附和增殖能力都随基质硬度的增加而增强。此外,除去正调控机制,已有研究显示,肿瘤微环境通过调节基质硬度的途径参与肿瘤干细胞的失巢凋亡抵抗,从而导致癌细胞代谢紊乱和死亡[74]。在材料孔隙方面也有相关报道,在12孔胶原涂层聚四氟乙烯模拟的上部腔室中,0.7 mm孔上培养的牙龈间充质干细胞的增殖量明显升高[75]。
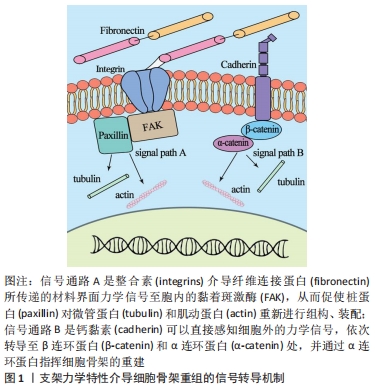
2.4 基质力学微环境对细胞分化的调控机制 细胞分化的本质是特定基因在表达时呈现的时空选择性(差异性),即机体在不同发育阶段的同种细胞或同一发育阶段的不同细胞中合成特异的蛋白质或RNA[76]。各类干细胞核内都含有机体的全套基因,但在表达层面存在差异,这导致了干细胞朝多个谱系分化的现象。当材料界面力学信号传递至细胞质膜时,细胞通过膜蛋白接收、胞内蛋白转导的形式以响应微环境的信号,而此过程便会致使分化相关基因的表达发生改变,实现干细胞的多向分化。根据目前的报道,不同硬度的基质可诱导全能或多能干细胞所分泌的分化相关蛋白差异性表达,致使此类干细胞朝不同谱系分化。对于脂肪干细胞而言,其质膜上镶嵌的整合素能将外界力学信号传递至质膜内表面的黏着斑激酶及其辅助蛋白——桩蛋白,同时这两种蛋白可将信号转递至质膜内表面附着的黏着斑蛋白处,黏着斑蛋白也可独立感知细胞外界的力学信号,并将其传递给胞内一种与信号转导相关的β连环蛋白,进而β连环蛋白通过核移位将信号传递给其下游蛋白,如淋巴增强子结合因子1 (lymphoid enhancer binding factor 1,LEF-1)和T细胞转录因子1 (T cell-specific transcription factor-1,TCF-1)等,往往这些下游蛋白能结合至分化相关基因,如血管内皮生长因子A的启动子区域,从而加强特定基因的表达,促进细胞分化,见图4。

就目前研究而言,多类干细胞分化受力学信号因素影响的情况已被证实,在此将根据干细胞种类分类进行阐述:①脂肪干细胞:在材料硬度方面,Xie等[77]研究了脂肪干细胞在软硬基质上分别向脂肪和成骨分化的信号转导机制;在其另一实验中,阐明了基质硬度调节血管内皮生长因子的表达,从而促进脂肪干细胞向血管分化的原理。DU等[78]也表明脂肪干细胞向血管生成的分化依赖于微环境的特性,包括生物物理因子如基质硬度等。DUAN等[79]表明,脂肪干细胞的谱系分化与周围微环境中存在的生物物理和生化线索(如基质刚度、细胞几何形状和黏附配体)密切相关,且通过材料力学信号可以激活基因表达使脂肪干细胞直接向成骨分化,即激活碱性磷酸酶、骨保护素、骨钙素、大麻素Ⅰ型受体、大麻素Ⅱ型受体、核因子κB受体激活因子配体和细胞外基质蛋白组分(即胶原蛋白、弹性蛋白等)等。在其他材料力学信号方面,PARK等[80]将脂肪干细胞种植在弹性纳米纤维支架上,并利用分化因子和力学协同作用诱导其分化为平滑肌细胞。他们发现静态培养组α-SMA和MHC基因的表达并没有受到分化因子的影响,而力学培养组α-SMA和MHC基因的表达受到了分化因子的影响。此外,近期报道显示,体外扩容装置产生的外力会增加脂肪组织的硬度,从而影响SD大鼠脂肪干细胞的分化。外力的大小可以根据特定时间段所需的组织刚度来改变,以促进长期的脂肪组织再生[81]。②间充质细胞:研究在材料硬度、拓扑结构等方面均有涉及。已有越来越多的证据表明,模拟不同组织微环境的基体弹性或刚度,包括神经元、脂肪组织、软骨和松质骨的生物力学特征,能够影响间充质干细胞分化成各种组织细胞,微环境刚度可以指导间充质干细胞向不同谱系分化,将骨髓间充质干细胞暴露于低、中、高刚度的基质中,将定向分化为脂肪系、内皮系、肌系和成骨系。同时也有研究指出,随着黏附性和硬度的提高,间充质干细胞的软骨分化会得到促进[82-86]。夏雨涵[87]还发现在不同基质硬度上,人脐带间充质干细胞的分化发生了明显变化:细胞在软基质上形态多呈现为短柱形,类似于肌细胞;而在硬基质上形态多呈现多角形,类似于成骨细胞。此外,WANG等[88]发现可注射、可降解水凝胶具有可调节的力学性能-硬度,该水凝胶可以用于刺激人骨髓间充质干细胞在三维培养中的神经分化。最新研究也正不断完善分子信号机制,如在骨髓间充质干细胞分化过程中,Integrin-α5/PI3K/Akt和Wnt/β-catenin信号通路发挥着重要的调节作用,表明高硬度的基质不仅能够调控细胞的自我更新能力,还能够诱导细胞进行成骨分化[89]。在基质拓扑结构方面,贺云飞等[90]通过构建3种结构不同的纳米纤维支架,即取向纳米纤维支架、取向纳米纱支架、三维多孔纳米纤维支架发现,骨髓间充质干细胞分别在取向纳米纤维支架、取向纳米纱支架上呈类成纤维细胞样生长,在三维多孔纳米纤维支架上呈类软骨细胞样生长。在孔隙大小和密度方面,WANG等[91]表明孔隙密度、大小和排列方式可联合胞外基质形貌共同影响间充质干细胞的软骨与成骨分化,然而这种影响在转化生长因子1作用下可被掩盖。肖倩茹[92]则将表面为纳米孔洞的反蛋白石结构和多孔网状拉伸结构作为支架的拓扑结构研究其对骨髓间充质干细胞分化的影响,研究结果表明,反蛋白结构和多孔网状拉伸结构可以诱导间充质干细胞向成骨细胞分化,并且这种诱导效应与结构表面纳米孔洞的孔径正相关,但当结构拉伸倍数太大时,成骨诱导效应反而会降低,说明拓扑结构影响干细胞分化存在阈值。而闫峰等[93]研究也证实,壳聚糖支架的孔隙大小和孔隙率对间充质干细胞神经分化具有重要影响,为未来神经系统疾病的治疗提供了新的方向。张述寅等[94]则发现,在碱性溶液中对Ti-6Al-7Nb合金进行电化学阳极氧化处理可形成薄的纳米孔层(厚度< 100 nm),由此得到的亲水表面纳米形貌孔径为10-100 nm,其与未经处理(无纳米孔)的合金相比,可增强人骨髓间充质干细胞的碱性磷酸酶活性、Runx2转录因子以及各种成骨标志物的表达。③胚胎干细胞:相关报道也都集中于材料硬度的影响。PRZYBYLA等[95]研究表明,在胞质信号转导过程中,在硬基质上Wnt/β-catenin信号表达减弱,从而导致人类胚胎干细胞向中胚层分化。也有研究证实,将人胚胎干细胞在水凝胶囊中培养,囊膜硬度的增加会促进内胚层中后期的分化,而硬度的增加强烈抑制了胰祖细胞方向的分化进程[96]。此外,研究胚胎干细胞对力学刺激的应答发现,血管细胞群的出现与特定的水凝胶硬度一致:内皮细胞谱系的出现则偏向于较软的材料,平滑肌细胞谱系的出现更偏向于坚硬的材料[97]。④牙相关干细胞:在材料硬度方面,BAI等[98]论述了材料硬度如何影响牙乳头细胞的牙源性分化;QU等[99]也发现,与低刚度支架上柔软的髓样组织相比,高刚度时用于皮下植入的支架可促进牙髓干细胞分化形成矿化组织;还有文献报道,与硬度最软的表面相比,最硬的表面更有利于人脱落乳牙干细胞成骨分化的发生[100];此外,VIALE-BOURONCLE等[101]发现软基质支持人牙囊细胞的成骨分化;最近研究显示,随着基质硬度的增加,牙髓干细胞中与成骨/牙本质分化相关的蛋白标记表达也显著增加,包括碱性磷酸酶、骨钙素、骨桥蛋白、Runx2、骨形态发生蛋白2、牙本质唾液磷蛋白和牙基质蛋白[102]。在材料形貌方面,HU等[103]研究探索了种植体表面微/纳米分级形貌对牙周膜干细胞生物学行为的调控作用,并证实了选择性激光熔融技术结合碱热处理(selective laser melting fully melting-alkali-heat treatment,SLM-AHT)微/纳米分级形貌钛表面具有良好的诱导牙周膜干细胞体外成骨分化的能力,该表面可以促进细胞骨架纤维型肌动蛋白的聚集,激活MAPK信号通路,从而促进PDZ结合基序转录共激活因子(transcriptional co-activator with PDZ-binding motif,TAZ)的核易位、激活,进而促进牙周膜干细胞成骨分化、抑制其成脂分化。在外力作用方面,压缩力还会促进牙周膜干细胞前列腺素E2的分泌增加、核因子κB受体激活因子配体的表达上调和破骨细胞的增多[104];模拟咀嚼力可诱导人牙髓基质细胞的成骨分化[105];微重力则能够导人牙周膜干细胞的成骨分化、促进增殖以及成骨蛋白碱性磷酸酶、Ⅰ型胶原和骨钙素水平提高[106];而多参数流动剪切力也会加强牙周膜干细胞的成骨分化,提高碱性磷酸酶、骨桥蛋白、淋巴增强因子1的表达[107]。⑤神经嵴干细胞:其研究主要集中于材料硬度的影响。ZHU等[108]研究显示,基质刚度在体内可以调节神经嵴干细胞向平滑肌系和胶质系分化。Li等[109]则表明,与软基质相比,硬基质可以促进人诱导多功能干细胞来源神经嵴干细胞向平滑肌细胞分化。⑥其他细胞:在粗糙度方面,FAIA-TORRES等[110]通过构造粗糙梯度基质的方式,确定了粗糙度对干细胞成骨命运分化的影响。在材料硬度方面,?WIERCZEK-LASEK 等[111]发现,弹性模量高于生理骨骼肌组织的聚二甲基硅氧烷材料不会干扰小鼠成肌细胞的分化,因此聚二甲基硅氧烷可作为肌肉组织工程的生物材料,其中弹性模量在2.5-4.0 MPa范围内的聚二甲基硅氧烷材料不影响其分化效率;也有报道称,YAP可能是AT1R下游的重要信号分子,在介导基质硬度诱导的心脏成纤维细胞分化过程中起重要作用[112]。CHU等[113]则以电纺聚醚碳酸酯聚氨酯脲为支架材料,对于纤维环起源细胞,随着支架硬度的增加,YAP会移位到细胞核中,但在硬度较小的支架上,YAP主要以磷酸化的形式保留在细胞的胞浆内,抑制YAP的核移位可下调肌腱/韧带相关基因的表达,反之则可上调软骨相关基因的表达。KOUROUKLIS等[114]还观察到双潜能小鼠胚胎肝祖细胞的肝细胞分化主要受细胞外基质成分的调节,而胆管细胞的分化则受细胞外基质蛋白和硬度特性的协同影响。此外,AGUILAR等[115]在三维甲基纤维素培养基中培养骨髓造血干细胞,当基质硬度与骨髓相似时,培养基中产生了形态与在体相似度极高的巨核细胞。
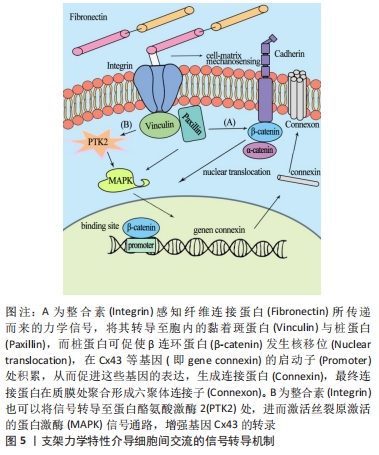
2.5 基质力学微环境调控细胞间交流的机制 细胞间的信息交流是细胞生命活动的重要组成部分。而细胞交流的结构基础是细胞接触和连接,微环境的力学信号通过改变细胞间的连接与信号传递形式实现对细胞交流的影响。通讯连接中的间隙连接与突触便是细胞交流的两大主要途径。间隙连接是相邻质膜不直接相连,存在2.0-3.0 nm的空隙,通过两个连接子(每个连接子由6个相似的连接蛋白六聚体组成)在胞外对接形成通道,允许cAMP、Ca2+等信号分子经通道进入到相邻细胞[116]。关于化学物质调控间隙连接产生的报道已有不少,如转化生长因子β1可与其Ⅰ、Ⅱ型受体结合,进而将信号传导至下游蛋白Smad 2,3,4或p-ERK处,从而通过这两种蛋白的核移位调节间隙连接相关基因如panx1和Cx43的转录,实现对细胞交流的影响[117-120]。而关于基质力学信号的调节还鲜有报道,但早在20世纪90年代就有报道显示,细胞外基质通过其理化特性决定着人颗粒细胞的缝隙连接程度[121],目前较为清晰的研究表明基质力学信号对间隙连接的影响是通过整合素来实现的。细胞外的力学信号经细胞膜上的整合素转导至胞内,整合素在胞内与黏着斑蛋白相连,而黏着斑蛋白通常伴有辅助蛋白-桩蛋白,桩蛋白进而调节Cx43装配或将信号传递至下游β连环蛋白,促使β连环蛋白核移位,结合于基因Cx43的启动子处,以两种途径的信号转导共同调节间隙连接[122-123]。也有最新的基于人牙根尖乳头干细胞的研究揭示,在整合素avβ3的介导下,细胞外材料界面的硬度力学信号可传递至质膜内表面的蛋白酪氨酸激酶2处,该激酶既可以通过将信号传递至非受体型蛋白酪氨酸激酶Src直接对Cx43蛋白进行组装调控;还能将信号通过磷酸化转导至MAPK信号通路,进而加强Cx43基因的转录,增强细胞间的间隙连接[124],见图5。
以突触为主的细胞交流,其研究主要集中在神经细胞方面。在体内神经冲动是单向传导的,当神经冲动通过轴突传递到突触小体时,突触前膜对钙离子的通透性增加,突触间隙中的钙离子涌入突触小体内,促使突触小泡与突触前膜紧密融合,将小泡内的神经递质释放至突触间隙中,并经过扩散到达突触后膜,立即与突触后膜上的受体结合,并且改变突触后膜通道对离子的通透性,引起突触后膜发生兴奋性或抑制性的变化。根据研究团队对小鼠海马神经元的研究,硬基底上培养的海马神经元细胞神经网络中同步自发性的钙离子振荡的振幅和频率都升高,兴奋性和抑制性细胞的突触连接都加强;硬基底上兴奋性突触后膜的电流增强(抑制性不变),且对脉冲易化减弱,而对脉冲抑制不变,这意味着含有神经递质的囊泡释放概率升高,硬基底增强了突触间兴奋性神经递质的传递[125]。
现有的关于受材料力学性质影响的细胞交流的报道还较少,在此将从成体细胞和干细胞两方面进行论述。成体细胞可大致分为以下3类。①神经元:从材料硬度的角度出发,PREVITERA等[126]表明,与生长在软基质上的神经元相比,在相同细胞数量下,培养在较硬基质上的神经元显示出更多的分支,这反映出基质硬度对细胞交流的影响。此外,机械拉伸皮质神经元可显著降低神经元自发性的钙离子振荡和兴奋性突触后膜电流的产生[127]。②内皮细胞:相关研究主要集中于材料硬度的影响。CHEN等[128]阐明了基质刚度如何调节内皮细胞和单核细胞之间的相互作用,即基质硬度可调节单核细胞与内皮细胞间的黏附作用。还有报道显示,在硬基质上内皮细胞因子蛋白激酶C的激活可增加内皮单层细胞活性的产生,然而这种氧化应激会影响、降低黏附连接的完整性,减弱细胞间交流[129]。此外,基质力学信号可通过β1整合素改变纤维型肌动蛋白的构象,重组紧密连接蛋白来增加血管内皮细胞的通透性,并涉及MLC信号通路[130]。③其他细胞:材料界面力学刺激导致细胞产生的ATP是内侧副韧带细胞内钙含量升高的主要介质,而嘌呤感受器的激活可能进一步放大该信号,以活化和召集更多的细胞对力学刺激做出反应[131]。此外,林雨[132]表明,软基质上肝实质细胞间易形成边-边接触的切向连接,而在硬基质上,细胞间易形成辐射状的细胞连接,基质硬度通过调节细胞的连接方式影响细胞通讯。
有关干细胞交流所受力学信号影响的报道主要集中于材料种类、材料硬度与外力种类3方面。在材料种类方面,相对于塑料表面的组织培养,在石墨烯上培养神经干细胞可增强细胞内同步自发的钙离子振荡和突触后膜电流,然而其具体机制尚不明晰[133]。在材料硬度方面,基质硬度会影响聚乙烯醇水凝胶上骨髓间充质细胞和肝细胞的信息交流[134];此外,在较硬的聚丙烯酰胺凝胶基质上培养时,人间充质干细胞中自发的钙离子振荡变得更加频繁,当从基质的软区域向硬区域迁移时,内皮细胞中钙离子的振荡会增强,从而影响细胞通讯[135]。在外力作用方面,循环拉伸应力可增强人骨髓间充质干细胞在各向异性胶原-糖胺聚糖支架中的Smad2/3活化,而Smad信号通路可介导缝隙连接的形成,从而促进细胞交流[136]。
2.6 基质力学微环境对细胞其他功能的调控与其潜在的机制 除上述细胞外环境对细胞生理活动的影响外,对于特定种类的细胞,基质也会对其特有的功能产生影响,但无一例外,这种影响都遵循着信号传导的模式进行,在此将从成体细胞与干细胞两方面进行阐述。在成体细胞中,可根据力学信号的种类大致分为5个角度。①材料界面的粗糙度:已有文献表明,材料表面的粗糙度会影响细胞功能[59,75],特别是在成骨过程中,表面粗糙度是调控骨整合的重要参数之一,经深入研究发现,基质粗糙的表征进一步模拟了骨表面的物理线索,强调了在骨吸收过程中材料粗糙度对破骨细胞活性影响的重要性[59]。②材料颗粒大小:以微孔退火颗粒水凝胶和镶嵌纳米级再生丝素蛋白的聚乙烯醇膜为基质材料时,TRUONG等[58]和陈忠敏等[137]分别发现,在颗粒较大材料上培养的人皮肤成纤维细胞中基因转移的效果最佳;而在颗粒大小较均匀时,仓鼠卵巢细胞的生长性能最佳,即具有最快的生长速度。③材料磁性程度:CORBIN等[138]通过在聚二甲基硅氧烷弹性体中添加磁性夹杂物,通过使用磁梯度来研究心肌细胞对动态硬化或软化的短期响应,如应力纤维形成、Yes相关蛋白转位、肌节组织的变化、组织修复、病理适应和细胞死亡等,这对更真实地模拟体内物理微环境的变化具有重要意义。④受力类型:不同的受力对同一种细胞有着差异性的作用,牵张力会抑制牙周膜细胞Ⅰ型胶原、骨钙素分泌,降低碱性磷酸酶的活性和增强核因子κB的表达[139-140];而周期性拉伸力则会使牙周膜细胞中的转化生长因子β、骨保护素表达上调,从而抑制牙周膜细胞活性[141-142]。⑤材料界面硬度:生理状态下,心肌细胞的成熟、肌节组构、自我更新、钙离子活动和同步协调性收缩等,都受到材料基质硬度的调控,由此可见,物理微环境力学信号对心肌细胞正常功能维持的重要性;如不同硬度的水凝胶会影响力学敏感转录因子YAP在成年人心肌细胞中的亚细胞定位,进而影响细胞凋亡或肿瘤发生的进程[143-145]。关于其他成体细胞如神经细胞和肝细胞的研究也有了新的进展,ALLEN等[146]对于成体细胞的研究显示,通过模拟不同组织微环境的底物硬度,如模仿神经组织、脂肪组织、软骨、松质骨或血管的基质硬度,能够影响细胞终末的表达和功能化;目前较清晰的是,在皮质神经元培养基中,与脑组织硬度相似的基质更倾向于刺激神经元的生长而非神经胶质细胞[147]。此外,肝细胞的组织培养已成为肝衰竭修复治疗中一种极具潜力的治疗方式,AGUILAR等[148]和DEEGAN等[149]研究揭示,材料界面硬度可诱导肝星状细胞的活化及细胞外基质蛋白的分泌,而原代肝细胞同样也受到基质硬度的影响,其黏附、功能标志物如白蛋白和肝细胞核因子的表达都与基质硬度息息相关。除却上述材料硬度对细胞的影响,细胞染色体区域和基因位点的可视化、极化重定向、外基质基因mRNA的表达,乃至凋亡也都与这一力学信号相关联。由此可见,物理微环境力学信号对细胞的影响因细胞类型而异并深入至细胞生理活动的各个方面[150-152]。病理状态下,胞外材料界面硬度依旧是介导细胞活动的重要因素。最新研究显示,在颗粒大小和表面电荷保持不变的情况下,阳离子明胶纳米颗粒的硬度决定了RNAi在髓系白血病细胞中的作用效率[153];而在癌细胞中,高硬度的无序自组装肽凝胶基质增强了乳腺癌细胞的生物活性;经深入研究发现,材料硬度可通过调节整合素连接激酶介导的YAP激活来控制乳腺癌细胞的耐药性[154-155]。
有关干细胞的研究,可大致从以下3方面对材料力学信号的影响进行阐述。①材料界面的拓扑结构:GRAZIANO 等[156]报道了牙髓干细胞在具有凹面几何形状的基质上具有分化速度快、细胞活性高和基质形成快等方面的优越性,证实了支架拓扑结构在组织工程中的重要性。②受力类型:CHAN 等[157]以小鼠囊胚来模拟充液腔,发现管腔压力和大小的改变会影响滋养外胚层细胞的分裂模式,从而影响细胞的分配和命运,揭示了腔内压力和其力学特性如何在组织尺度上控制胚胎的大小,这也与细胞尺度上的细胞定位和细胞命运相关。③材料界面硬度:干细胞的成熟、特定蛋白的表达都与材料界面的硬度相关,如小鼠骨髓祖细胞向巨核细胞的成熟、巨核细胞染色体倍性和前血小板形成的增强、牙髓基质干细胞的矿化作用、人骨髓间充质干细胞中参与蛋白激酶B磷酸化调控的细胞移动抑制因子的表达、造血干细胞中基质金属蛋白酶及金属蛋白酶组织抑制因子的表达;综上所述,物理微环境对干细胞的影响是特异而多元的,又因为干细胞常常作为组织工程中的种子细胞,因此,对干细胞应答的深入研究对未来规避力学信号在组织工程中的负面影响具有长远意义[158-159]。

| [1] 郭树忠,熊猛.组织工程组织发展的前景与面临的问题[J].西北国防医学杂志,2004,25(1):1-2. [2] 王佃亮.组织工程的诞生与发展——组织工程连载之一[J].中国生物工程杂志,2014,34(5):122-129. [3] 杨志明.组织工程的发展趋势[J].中国修复重建外科杂志,2003, 17(2):5-6. [4] 徐国恒.细胞骨架——微管[J].生物学通报,2005,40(5):21-22. [5] 徐国恒.细胞骨架——肌动蛋白纤维[J].生物学通报,2005,40(2):43. [6] XIE J, ZHANG D, LING Y, et al. Substrate elasticity regulates vascular endothelial growth factor A (VEGFA) expression in adipose-derived stromal cells: Implications for potential angiogenesis. Colloids Surf B Biointerfaces. 2019;175:576-585. [7] 周广东.“软骨组织工程种子细胞的研究进展”点评[J].中国眼耳鼻喉科杂志,2020,20(1):7-8,12. [8] 郭学彦.关节软骨组织再生修复技术新进展[J].中国当代医药, 2012,19(22):28-29,31. [9] ZHANG T, GONG T, XIE J, et al. Softening Substrates Promote Chondrocytes Phenotype via RhoA/ROCK Pathway. ACS Appl Mater Interfaces. 2016;8(35):22884-22891. [10] SCHUH E, KRAMER J, ROHWEDEL J, et al. Effect of matrix elasticity on the maintenance of the chondrogenic phenotype. Tissue Eng Part A. 2010;16(4):1281-1290. [11] CUI Z, YANG BF, LI RK. Application of Biomaterials in Cardiac Repair and Regeneration. Engineering. 2016;2(1):141-148. [12] JACOT JG, MCCULLOCH AD, OMENS JH. Substrate stiffness affects the functional maturation of neonatal rat ventricular myocytes. Biophys J. 2008;95(7):3479-3487. [13] WANG PY, YU J, LIN JH, et al. Modulation of alignment, elongation and contraction of cardiomyocytes through a combination of nanotopography and rigidity of substrates. Acta Biomater. 2011;7(9): 3285-3293. [14] SHAPIRA-SCHWEITZER K, SELIKTAR D. Matrix stiffness affects spontaneous contraction of cardiomyocytes cultured within a PEGylated fibrinogen biomaterial. Acta Biomater. 2007;3(1):33-41. [15] ODEDRA D, CHIU L, REIS L, et al. Cardiac Tissue Engineering. In: Burdick JA, Mauck RL. Biomaterials for Tissue Engineering Applications. Vienna: Springer, 2011:421-456. [16] HAJI AH. Cells adapt their shapes with their experienced stiffness: A geometrical approach to differentiation. J Theor Biol. 2020;486:110086. [17] XIE J, ZHANG Q, ZHU T, et al. Substrate stiffness-regulated matrix metalloproteinase output in myocardial cells and cardiac fibroblasts: implications for myocardial fibrosis. Acta Biomater. 2014;10(6):2463-2472. [18] FORTE G, PAGLIARI S, EBARA M, et al. Substrate stiffness modulates gene expression and phenotype in neonatal cardiomyocytes in vitro. Tissue Eng Part A. 2012;18(17-18):1837-1848. [19] BASTOUNIS EE, YEH YT, THERIOT JA. Subendothelial stiffness alters endothelial cell traction force generation while exerting a minimal effect on the transcriptome. Sci Rep. 2019;9(1):18209. [20] KAYAL C, MOEENDARBARY E, SHIPLEY RJ, et al. Mechanical Response of Neural Cells to Physiologically Relevant Stiffness Gradients. Adv Healthc Mater. 2020;9(8):e1901036. [21] TOWORFE GK, COMPOSTO RJ, ADAMS CS, et al. Fibronectin adsorption on surface-activated poly(dimethylsiloxane) and its effect on cellular function. J Biomed Mater Res A. 2004;71(3):449-461. [22] WU M, HE J, REN X, et al. Development of functional biointerfaces by surface modification of polydimethylsiloxane with bioactive chlorogenic acid. Colloids Surf B Biointerfaces. 2014;116:700-706. [23] RAZAFIARISON T, SILVÁN U, MEIER D, et al. Surface-Driven Collagen Self-Assembly Affects Early Osteogenic Stem Cell Signaling. Adv Healthc Mater. 2016;5(12):1481-1492. [24] ENGLER AJ, SEN S, SWEENEY HL, et al. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677-689. [25] MALDONADO M, WONG LY, ECHEVERRIA C, et al. The effects of electrospun substrate-mediated cell colony morphology on the self-renewal of human induced pluripotent stem cells. Biomaterials. 2015; 50:10-19. [26] XIAO Z, BAUDRY J, CAO L, et al. Polycystin-1 interacts with TAZ to stimulate osteoblastogenesis and inhibit adipogenesis. J Clin Invest. 2018;128(1):157-174. [27] KIM C, YOUNG JL, HOLLE AW, et al. Stem Cell Mechanosensation on Gelatin Methacryloyl (GelMA) Stiffness Gradient Hydrogels. Ann Biomed Eng. 2020;48(2):893-902. [28] MELICA ME, LA REGINA G, PARRI M, et al. Substrate Stiffness Modulates Renal Progenitor Cell Properties via a ROCK-Mediated Mechanotransduction Mechanism. Cells. 2019;8(12):1561. [29] SUN X, TUNG W, WANG W, et al. The effect of stiffness variation of electrospun fiber meshes of multiblock copolymers on the osteogenic differentiation of human mesenchymal stem cells. Clin Hemorheol Microcirc. 2019;73(1):219-228. [30] BRENNAN CM, EICHHOLZ KF, HOEY DA. The effect of pore size within fibrous scaffolds fabricated using melt electrowriting on human bone marrow stem cell osteogenesis. Biomed Mater. 2019;14(6):065016. [31] WEI SC, FATTET L, TSAI JH, et al. Matrix stiffness drives epithelial-mesenchymal transition and tumour metastasis through a TWIST1-G3BP2 mechanotransduction pathway. Nat Cell Biol. 2015;17(5):678-688. [32] 陈立伟,张雨晨,王子秋,等.Twist1蛋白与肿瘤[J].中国生物化学与分子生物学报,2017,33(12):40-47. [33] MIRON-MENDOZA M, SEEMANN J, GRINNELL F. The differential regulation of cell motile activity through matrix stiffness and porosity in three dimensional collagen matrices. Biomaterials. 2010;31(25): 6425-6435. [34] LIM J, CHOI A, KIM HW, et al. Constrained Adherable Area of Nanotopographic Surfaces Promotes Cell Migration through the Regulation of Focal Adhesion via Focal Adhesion Kinase/Rac1 Activation. ACS Appl Mater Interfaces. 2018;10(17):14331-14341. [35] PHAM JT, XUE L, DEL CAMPO A, et al. Guiding cell migration with microscale stiffness patterns and undulated surfaces. Acta Biomater. 2016;38:106-115. [36] NASROLLAHI S, WALTER C, LOZA AJ, et al. Past matrix stiffness primes epithelial cells and regulates their future collective migration through a mechanical memory. Biomaterials. 2017;146:146-155. [37] ROY NH, MACKAY JL, ROBERTSON TF, et al. Crk adaptor proteins mediate actin-dependent T cell migration and mechanosensing induced by the integrin LFA-1. Sci Signal. 2018;11(560):eaat3178. [38] SONG KH, HEO SJ, PEREDO AP, et al. Influence of Fiber Stiffness on Meniscal Cell Migration into Dense Fibrous Networks. Adv Healthc Mater. 2020;9(8):e1901228. [39] EHRBAR M, SALA A, LIENEMANN P, et al. Elucidating the role of matrix stiffness in 3D cell migration and remodeling. Biophys J. 2011; 100(2):284-293. [40] XIE J, ZHOU CC, ZHANG DM, et al. Compliant substratum changes osteocyte functions: the role of ITGB3/FAK/β-catenin signaling matters. ACS Appl Bio Mater. 2018;1(3):792-801. [41] FENG S, ZHOU L, ZHANG Y, et al. Mechanochemical modeling of neutrophil migration based on four signaling layers, integrin dynamics, and substrate stiffness. Biomech Model Mechanobiol. 2018;17(6): 1611-1630. [42] KUO CH, XIAN J, BRENTON JD, et al. Complex stiffness gradient substrates for studying mechanotactic cell migration. Adv Mater. 2012;24(45):6059-6064. [43] MORI H, TAKAHASHI A, HORIMOTO A, et al. Migration of glial cells differentiated from neurosphere-forming neural stem/progenitor cells depends on the stiffness of the chemically cross-linked collagen gel substrate. Neurosci Lett. 2013;555:1-6. [44] PATHAK A. Modeling and predictions of biphasic mechanosensitive cell migration altered by cell-intrinsic properties and matrix confinement. Phys Biol. 2018;15(6):065001. [45] GEIGER F, RÜDIGER D, ZAHLER S, et al. Fiber stiffness, pore size and adhesion control migratory phenotype of MDA-MB-231 cells in collagen gels. PLoS One. 2019;14(11):e0225215. [46] WANG M, CHENG B, YANG Y, et al. Microchannel Stiffness and Confinement Jointly Induce the Mesenchymal-Amoeboid Transition of Cancer Cell Migration. Nano Lett. 2019;19(9):5949-5958. [47] DA SILVA J, LAUTENSCHLÄGER F, SIVANIAH E, et al. The cavity-to-cavity migration of leukaemic cells through 3D honey-combed hydrogels with adjustable internal dimension and stiffness. Biomaterials. 2010; 31(8):2201-2208. [48] WANG M, CHENG B, YANG YW, et al. The matrix stiffness and physical confinement of hydrogel microchannel jointly induce the mesenchymal-amoeboid transition for cancer cell migration. Journal of Medical Biomechanics. 2019;34(S1):137-138. [49] WANG HR, ZHANG Y, OZDAMAR B, et al. Regulation of cell polarity and protrusion formation by targeting RhoA for degradation. Science. 2003;302(5651):1775-1779. [50] MCKENZIE AJ, HICKS SR, SVEC KV, et al. The mechanical microenvironment regulates ovarian cancer cell morphology, migration, and spheroid disaggregation. Sci Rep. 2018;8(1):7228. [51] MIERKE CT. The matrix environmental and cell mechanical properties regulate cell migration and contribute to the invasive phenotype of cancer cells. Rep Prog Phys. 2019;82(6):064602. [52] LIN C, TAO B, DENG Y, et al. Matrix promote mesenchymal stromal cell migration with improved deformation via nuclear stiffness decrease. Biomaterials. 2019;217:119300. [53] BATTAGLIA RA, DELIC S, HERRMANN H, et al. Vimentin on the move: new developments in cell migration. F1000Res. 2018;7:F1000 Faculty Rev-1796. [54] 魏海彬,花丽洁,张鹏,等.细胞外基质在中枢神经系统细胞迁移调控中的作用[J].全科口腔医学电子杂志,2018,5(34):42-43. [55] RAUSCH V, HANSEN CG. The Hippo Pathway, YAP/TAZ, and the Plasma Membrane. Trends Cell Biol. 2020;30(1):32-48. [56] 邵伟华,李晋,龚炜韬,等.Hippo-TAZ/YAP信号通路在头颈部鳞状细胞癌中的研究进展[J].口腔医学,2020,40(1):71-77. [57] WANG R, THAYER P, GOLDSTEIN A, et al. Interaction of material stiffness and negative pressure to enhance differentiation of bone marrow-derived stem cells and osteoblast proliferation. J Tissue Eng Regen Med. 2020;14(2):295-305. [58] TRUONG NF, KURT E, TAHMIZYAN N, et al. Microporous annealed particle hydrogel stiffness, void space size, and adhesion properties impact cell proliferation, cell spreading, and gene transfer. Acta Biomater. 2019;94:160-172. [59] MITTAL A, KUMAR R, PARSAD D, et al. Cytomodulin-functionalized porous PLGA particulate scaffolds respond better to cell migration, actin production and wound healing in rodent model. J Tissue Eng Regen Med. 2014;8(5):351-363. [60] KUNZLER TP, DROBEK T, SCHULER M, et al. Systematic study of osteoblast and fibroblast response to roughness by means of surface-morphology gradients. Biomaterials. 2007;28(13):2175-2182. [61] GU Y, JI Y, ZHAO Y, et al. The influence of substrate stiffness on the behavior and functions of Schwann cells in culture. Biomaterials. 2012; 33(28):6672-6681. [62] MASTERTON S, AHEARNE M. Influence of polydimethylsiloxane substrate stiffness on corneal epithelial cells. R Soc Open Sci. 2019; 6(12):191796. [63] LAVALLEY DJ, ZANOTELLI MR, BORDELEAU F, et al. Matrix Stiffness Enhances VEGFR-2 Internalization, Signaling, and Proliferation in Endothelial Cells. Converg Sci Phys Oncol. 2017;3:044001. [64] SHAHEEN S, WAN Z, HANEEF K, et al. B cell mechanosensing: A mechanistic overview. Adv Immunol. 2019;144:23-63. [65] HASANOVA GI, NORIEGA SE, MAMEDOV TG, et al. The effect of ultrasound stimulation on the gene and protein expression of chondrocytes seeded in chitosan scaffolds. J Tissue Eng Regen Med. 2011;5(10):815-822. [66] MUI KL, BAE YH, GAO L, et al. N-Cadherin Induction by ECM Stiffness and FAK Overrides the Spreading Requirement for Proliferation of Vascular Smooth Muscle Cells. Cell Rep. 2015;10(9):1477-1486. [67] 贺小涛,李璇,夏宇,等.高硬度基质胶复合IL-4/SDF-1α调控巨噬细胞极化促进牙周组织内源性再生的研究[C].沈阳:2019年中华口腔医学会牙周病学专业委员会牙周病与植体周病新分类·新理论·新技术高峰论坛会议手册及论文汇编,2019:122-124. [68] 焦思萌,赵轩宇,宋丹,等.基底刚度对人宫颈癌HeLa细胞增殖及顺铂药物敏感性影响的体外研究[J].癌症进展,2019,17(22):2642-2644,2651. [69] 刘秋萍,田博仁,罗庆,等.基质刚度对肝癌细胞增殖及糖代谢的影响[J].医用生物力学,2019,34(2):133-138. [70] KALLI M, STYLIANOPOULOS T. Defining the Role of Solid Stress and Matrix Stiffness in Cancer Cell Proliferation and Metastasis. Front Oncol. 2018;8:55. [71] ARSHI A, NAKASHIMA Y, NAKANO H, et al. Rigid microenvironments promote cardiac differentiation of mouse and human embryonic stem cells. Sci Technol Adv Mater. 2013;14(2):025003. [72] CHITTETI BR, KACENA MA, VOYTIK-HARBIN SL, et al. Modulation of hematopoietic progenitor cell fate in vitro by varying collagen oligomer matrix stiffness in the presence or absence of osteoblasts. J Immunol Methods. 2015;425:108-113. [73] FIORETTA ES, FLEDDERUS JO, BAAIJENS FP, et al. Influence of substrate stiffness on circulating progenitor cell fate. J Biomech. 2012;45(5):736-744. [74] AYLA S, KARAHÜSEYINOGLUC S. Cancer Stem Cells, Their Microenvironment and Anoikis. Crit Rev Oncog. 2019;24(1):27-34. [75] GAMAL AY, AL-BERRY NN, HASSAN AA, et al. In vitro evaluation of the human gingival fibroblast/gingival mesenchymal stem cell dynamics through perforated guided tissue membranes: cell migration, proliferation and membrane stiffness assay. J Periodontal Res. 2017; 52(3):628-635. [76] 刘德培,梁植权.细胞分化与基因表达调控[J].生物工程进展,1994, 14(1):23-25. [77] XIE J, ZHANG D, ZHOU C, et al. Substrate elasticity regulates adipose-derived stromal cell differentiation towards osteogenesis and adipogenesis through β-catenin transduction. Acta Biomater. 2018;79:83-95. [78] DU J, CHEN X, LIANG X, et al. Integrin activation and internalization on soft ECM as a mechanism of induction of stem cell differentiation by ECM elasticity. Proc Natl Acad Sci U S A. 2011; 108(23):9466-9471. [79] DUAN W, HAQUE M, KEARNEY MT, et al. Collagen and Hydroxyapatite Scaffolds Activate Distinct Osteogenesis Signaling Pathways in Adult Adipose-Derived Multipotent Stromal Cells. Tissue Eng Part C Methods. 2017;23(10):592-603. [80] PARK IS, KIM SH, HEO DN, et al. Synergistic effect of biochemical factors and strain on the smooth muscle cell differentiation of adipose-derived stem cells on an elastic nanofibrous scaffold. J Biomater Sci Polym Ed. 2012;23(12):1579-1593. [81] LI Y, WU M, ZHANG Z, et al. Application of External Force Regulates the Migration and Differentiation of Adipose-Derived Stem/Progenitor Cells by Altering Tissue Stiffness. Tissue Eng Part A. 2019;25(23-24): 1614-1622. [82] SCHUH E, HOFMANN S, STOK K, et al. Chondrocyte redifferentiation in 3D: the effect of adhesion site density and substrate elasticity. J Biomed Mater Res A. 2012;100(1):38-47. [83] WAN W, CHENG B, ZHANG C, et al. Synergistic Effect of Matrix Stiffness and Inflammatory Factors on Osteogenic Differentiation of MSC. Biophys J. 2019;117(1):129-142. [84] WU L, MAGAZ A, DARBYSHIRE A, et al. Thermoresponsive Stiffness Softening of Hierarchically Porous Nanohybrid Membranes Promotes Niches for Mesenchymal Stem Cell Differentiation. Adv Healthc Mater. 2019;8(10):e1801556. [85] JIANG YC, JIAO HL, LEE MS, et al. Endogenous biological factors modulated by substrate stiffness regulate endothelial differentiation of mesenchymal stem cells. J Biomed Mater Res A. 2018;106(6):1595-1603. [86] ZHAN X. Effect of matrix stiffness and adhesion ligand density on chondrogenic differentiation of mesenchymal stem cells. J Biomed Mater Res A. 2020;108(3):675-683. [87] 夏雨涵.基质硬度通过Piezo1调控hUC-MSCs成心肌分化的研究[D].长春:吉林大学,2019. [88] WANG LS, CHUNG JE, CHAN PP, et al. Injectable biodegradable hydrogels with tunable mechanical properties for the stimulation of neurogenesic differentiation of human mesenchymal stem cells in 3D culture. Biomaterials. 2010;31(6):1148-1157. [89] 孙美玉.细胞外基质硬度诱导骨髓间充质干细胞成骨分化的分子机制研究[D].长春:吉林大学,2018. [90] 贺云飞,王爽,马俊.P38/AKT通路调控骨髓间充质干细胞在不同空间结构纳米纤维环支架中的定向分化[J].中国组织工程研究, 2020,24(10):1540-1546. [91] WANG J, XIE L, WANG X, et al. The effects of oyster shell/alpha-calcium sulfate hemihydrate/platelet-rich plasma/bone mesenchymal stem cells bioengineering scaffold on rat critical-sized calvarial defects. J Mater Sci Mater Med. 2020;31(11):96. [92] 肖倩茹.生物材料表面纳米结构促进干细胞向成骨细胞分化的作用研究[D].南京:东南大学,2015. [93] 闫峰,张越林,岳伟,等.体外构建纯化骨髓间充质干细胞与壳聚糖生物支架复合体的实验研究[J].中国现代神经疾病杂志,2014, 14(11):1000-1006. [94] 张述寅,胡开进,莫静珍,等.Ti-6Al-7Nb合金表面经HF酸蚀+阳极氧化处理后对骨髓间充质干细胞特性影响的研究[J].牙体牙髓牙周病学杂志,2013,23(5):310-314. [95] PRZYBYLA L, LAKINS JN, WEAVER VM. Tissue Mechanics Orchestrate Wnt-Dependent Human Embryonic Stem Cell Differentiation. Cell Stem Cell. 2016;19(4):462-475. [96] RICHARDSON T, BARNER S, CANDIELLO J, et al. Capsule stiffness regulates the efficiency of pancreatic differentiation of human embryonic stem cells. Acta Biomater. 2016;35:153-165. [97] WONG L, KUMAR A, GABELA-ZUNIGA B, et al. Substrate stiffness directs diverging vascular fates. Acta Biomater. 2019;96:321-329. [98] BAI M, XIE J, LIU X, et al. Microenvironmental Stiffness Regulates Dental Papilla Cell Differentiation: Implications for the Importance of Fibronectin-Paxillin-β-Catenin Axis. ACS Appl Mater Interfaces. 2018;10(32):26917-26927. [99] QU T, JING J, REN Y, et al. Complete pulpodentin complex regeneration by modulating the stiffness of biomimetic matrix. Acta Biomater. 2015; 16:60-70. [100] VIALE-BOURONCLE S, GOSAU M, KÜPPER K, et al. Rigid matrix supports osteogenic differentiation of stem cells from human exfoliated deciduous teeth (SHED). Differentiation. 2012;84(5):366-370. [101] VIALE-BOURONCLE S, VÖLLNER F, MÖHL C, et al. Soft matrix supports osteogenic differentiation of human dental follicle cells. Biochem Biophys Res Commun. 2011;410(3):587-592. [102] LIU N, ZHOU M, ZHANG Q, et al. Stiffness regulates the proliferation and osteogenic/odontogenic differentiation of human dental pulp stem cells via the WNT signalling pathway. Cell Prolif. 2018;51(2): e12435. [103] HU P, GAO Q, ZHENG H, et al. The Role and Activation Mechanism of TAZ in Hierarchical Microgroove/Nanopore Topography-Mediated Regulation of Stem Cell Differentiation. Int J Nanomedicine. 2021;16: 1021-1036. [104] KANZAKI H, CHIBA M, SHIMIZU Y, et al. Periodontal ligament cells under mechanical stress induce osteoclastogenesis by receptor activator of nuclear factor kappaB ligand up-regulation via prostaglandin E2 synthesis. J Bone Miner Res. 2002;17(2):210-220. [105] JI J, SUN W, WANG W, et al. The effect of mechanical loading on osteogenesis of human dental pulp stromal cells in a novel in vitro model. Cell Tissue Res. 2014;358(1):123-133. [106] 李石.模拟微重力环境对人牙周膜干细胞增殖分化的影响及其机制的研究[D].西安:第四军医大学,2009. [107] 张桂荣,陈罗萍,王雪.流体剪切力对人牙周膜细胞LEF-1 mRNA表达影响研究[J].中国实用口腔科杂志,2015,8(1):32-35. [108] ZHU Y, LI X, JANAIRO RRR, et al. Matrix stiffness modulates the differentiation of neural crest stem cells in vivo. J Cell Physiol. 2019; 234(5):7569-7578. [109] LI X, XU R, TU X, et al. Differentiation of Neural Crest Stem Cells in Response to Matrix Stiffness and TGF-β1 in Vascular Regeneration. Stem Cells Dev. 2020;29(4):249-256. [110] FAIA-TORRES AB, GUIMOND-LISCHER S, ROTTMAR M, et al. Differential regulation of osteogenic differentiation of stem cells on surface roughness gradients. Biomaterials. 2014;35(33):9023-9032. [111] ŚWIERCZEK-LASEK B, KEREMIDARSKA-MARKOVA M, HRISTOVA-PANUSHEVA K, et al. Polydimethylsiloxane materials with supraphysiological elasticity enable differentiation of myogenic cells. J Biomed Mater Res A. 2019;107(12):2619-2628. [112] NIU L, JIA Y, WU M, et al. Matrix stiffness controls cardiac fibroblast activation through regulating YAP via AT1 R. J Cell Physiol. 2020;235 (11):8345-8357. [113] CHU G, YUAN Z, ZHU C, et al. Substrate stiffness- and topography-dependent differentiation of annulus fibrosus-derived stem cells is regulated by Yes-associated protein. Acta Biomater. 2019;92:254-264. [114] KOUROUKLIS AP, KAYLAN KB, UNDERHILL GH. Substrate stiffness and matrix composition coordinately control the differentiation of liver progenitor cells. Biomaterials. 2016;99:82-94. [115] AGUILAR A, BOSCHER J, PERTUY F, et al. Three-Dimensional Culture in a Methylcellulose-Based Hydrogel to Study the Impact of Stiffness on Megakaryocyte Differentiation. Methods Mol Biol. 2018;1812:139-153. [116] 冯全义,吴泽志,蔡绍,等.间隙连接研究进展[J].四川生理科学杂志,2004,26(2):73-77. [117] LIU W, ZHANG D, LI X, et al. TGF-β1 facilitates cell-cell communication in osteocytes via connexin43- and pannexin1-dependent gap junctions. Cell Death Discov. 2019;5:141. [118] WANG Q, ZHOU C, ZHANG D, et al. The involvement of the ERK-MAPK pathway in TGF-β1-mediated connexin43-gap junction formation in chondrocytes. Connect Tissue Res. 2019;60(5):477-486. [119] WANG Q, ZHOU C, LI X, et al. TGF-β1 promotes gap junctions formation in chondrocytes via Smad3/Smad4 signalling. Cell Prolif. 2019;52(2):e12544. [120] LIU W, CUI Y, SUN J, et al. Transforming growth factor-beta1 up-regulates connexin43 expression in osteocytes via canonical Smad-dependent signaling pathway. Biosci Rep. 2018;38(6):BSR20181678. [121] ZHANG D, ZHOU C, WANG Q, et al. Extracellular Matrix Elasticity Regulates Osteocyte Gap Junction Elongation: Involvement of Paxillin in Intracellular Signal Transduction. Cell Physiol Biochem. 2018;51(3): 1013-1026. [122] KAMIOKA H, ISHIHARA Y, RIS H, et al. Primary cultures of chick osteocytes retain functional gap junctions between osteocytes and between osteocytes and osteoblasts. Microsc Microanal. 2007;13(2): 108-117. [123] ZHOU C, ZHANG D, DU W, et al. Substrate mechanics dictate cell-cell communication by gap junctions in stem cells from human apical papilla. Acta Biomater. 2020;107:178-193. [124] AMSTERDAM A, ROTMENSCH S, FURMAN A, et al. Synergistic effect of human chorionic gonadotropin and extracellular matrix on in vitro differentiation of human granulosa cells: progesterone production and gap junction formation. Endocrinology. 1989;124(4):1956-1964. [125] ZHANG QY, ZHANG YY, XIE J, et al. Stiff substrates enhance cultured neuronal network activity. Sci Rep. 2014;4:6215. [126] PREVITERA ML, LANGHAMMER CG, FIRESTEIN BL. Effects of substrate stiffness and cell density on primary hippocampal cultures. J Biosci Bioeng. 2010;110(4):459-470. [127] GOFORTH PB, REN J, SCHWARTZ BS, et al. Excitatory synaptic transmission and network activity are depressed following mechanical injury in cortical neurons. J Neurophysiol. 2011;105(5):2350-2363. [128] CHEN W, TIAN B, LIANG J, et al. Matrix stiffness regulates the interactions between endothelial cells and monocytes. Biomaterials. 2019;221:119362. [129] URBANO RL, SWAMINATHAN S, CLYNE AM. Stiff Substrates Enhance Endothelial Oxidative Stress in Response to Protein Kinase C Activation. Appl Bionics Biomech. 2019;2019:6578492. [130] IZAWA Y, GU YH, OSADA T, et al. β1-integrin-matrix interactions modulate cerebral microvessel endothelial cell tight junction expression and permeability. J Cereb Blood Flow Metab. 2018;38(4):641-658. [131] JONES BF, WALL ME, CARROLL RL, et al. Ligament cells stretch-adapted on a microgrooved substrate increase intercellular communication in response to a mechanical stimulus. J Biomech. 2005;38(8):1653-1664. [132] 林雨.基底硬度变化对肝细胞骨架构象及细胞连结方式的影响[D].重庆:重庆大学,2009. [133] TANG M, SONG Q, LI N, et al. Enhancement of electrical signaling in neural networks on graphene films. Biomaterials. 2013;34(27):6402-6411. [134] CAO XM, HUANG QP, CHEN SS. Bone marrow mesenchymal stem cells interactions with hepatocytes and hepatic stellate cells on different stiff substrates. Zhonghua Gan Zang Bing Za Zhi. 2019;27(6):424-429. [135] SHYY JY, CHIEN S. Role of integrins in endothelial mechanosensing of shear stress. Circ Res. 2002;91(9):769-775. [136] GRIER WG, MOY AS, HARLEY BA. Cyclic tensile strain enhances human mesenchymal stem cell Smad 2/3 activation and tenogenic differentiation in anisotropic collagen-glycosaminoglycan scaffolds. Eur Cell Mater. 2017;33:227-239. [137] 陈忠敏,郝雪菲,范开.镶嵌纳米级再生丝素蛋白聚乙烯醇膜的制备与促细胞生长性能[J].生物医学工程学杂志,2010,27(6):1292-1297. [138] CORBIN EA, VITE A, PEYSTER EG, et al. Tunable and Reversible Substrate Stiffness Reveals a Dynamic Mechanosensitivity of Cardiomyocytes. ACS Appl Mater Interfaces. 2019;11(23):20603-20614. [139] 刘加强,刘洪臣,房兵,等.周期性张应力对人牙周膜细胞生物学活性的影响[J].中华老年口腔医学杂志,2012,10(2):68-71. [140] RITTER N, MUSSIG E, STEINBERG T, et al. Elevated expression of genes assigned to NF-kappaB and apoptotic pathways in human periodontal ligament fibroblasts following mechanical stretch. Cell Tissue Res. 2007;328(3):537-548. [141] TSUJI K, UNO K, ZHANG GX, et al. Periodontal ligament cells under intermittent tensile stress regulate mRNA expression of osteoprotegerin and tissue inhibitor of matrix metalloprotease-1 and -2. J Bone Miner Metab. 2004;22(2):94-103. [142] KANZAKI H, CHIBA M, SATO A, et al. Cyclical tensile force on periodontal ligament cells inhibits osteoclastogenesis through OPG induction. J Dent Res. 2006;85(5):457-462. [143] SMITH AW, HOYNE JD, NGUYEN PK, et al. Direct reprogramming of mouse fibroblasts to cardiomyocyte-like cells using Yamanaka factors on engineered poly(ethylene glycol) (PEG) hydrogels. Biomaterials. 2013;34(28):6559-6571. [144] CROCINI C, WALKER CJ, ANSETH KS, et al. Three-dimensional encapsulation of adult mouse cardiomyocytes in hydrogels with tunable stiffness. Prog Biophys Mol Biol. 2020;154:71-79. [145] DURÁN-PASTÉN ML, CORTES D, VALENCIA-AMAYA AE, et al. Cell Culture Platforms with Controllable Stiffness for Chick Embryonic Cardiomyocytes. Biomimetics (Basel). 2019;4(2):33. [146] ALLEN JL, COOKE ME, ALLISTON T. ECM stiffness primes the TGFβ pathway to promote chondrocyte differentiation. Mol Biol Cell. 2012; 23(18):3731-3742. [147] GEORGES PC, MILLER WJ, MEANEY DF, et al. Matrices with compliance comparable to that of brain tissue select neuronal over glial growth in mixed cortical cultures. Biophys J. 2006;90(8):3012-3018. [148] AGUILAR A, PERTUY F, ECKLY A, et al. Importance of environmental stiffness for megakaryocyte differentiation and proplatelet formation. Blood. 2016;128(16):2022-2032. [149] DEEGAN DB, ZIMMERMAN C, SKARDAL A, et al. Stiffness of hyaluronic acid gels containing liver extracellular matrix supports human hepatocyte function and alters cell morphology. J Mech Behav Biomed Mater. 2015;55:87-103. [150] PRADHAN R, SENGUPTA K. Imaging Chromosome Territory and Gene Loci Positions in Cells Grown on Soft Matrices. Methods Mol Biol. 2019;2038:181-197. [151] CHEN DY, CREST J, STREICHAN SJ, et al. Extracellular matrix stiffness cues junctional remodeling for 3D tissue elongation. Nat Commun. 2019;10(1):3339. [152] ZHANG YH, ZHAO CQ, JIANG LS, et al. Substrate stiffness regulates apoptosis and the mRNA expression of extracellular matrix regulatory genes in the rat annular cells. Matrix Biol. 2011;30(2):135-144. [153] ZHANG W, HAN B, LAI X, et al. Stiffness of cationized gelatin nanoparticles is a key factor determining RNAi efficiency in myeloid leukemia cells. Chem Commun (Camb). 2020;56(8):1255-1258. [154] QIN X, LV X, LI P, et al. Matrix stiffness modulates ILK-mediated YAP activation to control the drug resistance of breast cancer cells. Biochim Biophys Acta Mol Basis Dis. 2020;1866(3):165625. [155] ASHWORTH JC, THOMPSON JL, JAMES JR, et al. Peptide gels of fully-defined composition and mechanics for probing cell-cell and cell-matrix interactions in vitro. Matrix Biol. 2020;85-86:15-33. [156] GRAZIANO A, D’AQUINO R, CUSELLA-DE ANGELIS MG, et al. Scaffold’s surface geometry significantly affects human stem cell bone tissue engineering. J Cell Physiol. 2008;214(1):166-172. [157] CHAN CJ, COSTANZO M, RUIZ-HERRERO T, et al. Hydraulic control of mammalian embryo size and cell fate. Nature. 2019;571(7763):112-116. [158] DATKO WILLIAMS L, FARLEY A, CUPELLI M, et al. Effects of substrate stiffness on dental pulp stromal cells in culture. J Biomed Mater Res A. 2018;106(7):1789-1797. [159] YUAN H, ZHOU Y, LEE MS, et al. A newly identified mechanism involved in regulation of human mesenchymal stem cells by fibrous substrate stiffness. Acta Biomater. 2016;42:247-257. [160] MARTINS A, ALVES DA SILVA ML, FARIA S, et al. The influence of patterned nanofiber meshes on human mesenchymal stem cell osteogenesis. Macromol Biosci. 2011;11(7):978-987. [161] DUPONT-GILLAIN CC, JACQUEMART I, ROUXHET PG. Influence of the aggregation state in solution on the supramolecular organization of adsorbed type I collagen layers. Colloids Surf B Biointerfaces. 2005;43(3-4):179-186. |
| [1] | 吴 聪, 贾全忠, 刘 伦. 成年关节软骨碎块化后转化生长因子β1表达与软骨细胞迁移的关系[J]. 中国组织工程研究, 2022, 26(8): 1167-1172. |
| [2] | 王 景, 熊 山, 曹 金, 冯林伟, 王 信. 白细胞介素3在骨代谢中的作用及机制[J]. 中国组织工程研究, 2022, 26(8): 1260-1265. |
| [3] | 肖 豪, 刘 静, 周 君. 脉冲电磁场治疗绝经后骨质疏松症的研究进展[J]. 中国组织工程研究, 2022, 26(8): 1266-1271. |
| [4] | 闻丹丹, 李 强, 沈才齐, 纪 哲, 金培生. 外用红色诺卡氏菌细胞壁骨架提高脂肪间充质干细胞活性修复糖尿病创面[J]. 中国组织工程研究, 2022, 26(7): 1038-1044. |
| [5] | 朱兵兵, 邓江华, 陈晶晶, 慕晓玲. 白细胞介素8受体可提高脐带间充质干细胞迁移和向损伤内皮的黏附[J]. 中国组织工程研究, 2022, 26(7): 1045-1050. |
| [6] | 罗小玲, 张 丽, 杨茂桦, 徐 洁, 徐晓梅. 柚皮素干预人牙周膜干细胞的成骨分化能力[J]. 中国组织工程研究, 2022, 26(7): 1051-1056. |
| [7] | 熊挺淋, 应梦慧, 张丽莎, 张晓刚, 杨 燕. 诱导多能干细胞分化的心肌细胞电生理特点[J]. 中国组织工程研究, 2022, 26(7): 1063-1067. |
| [8] | 王新民, 刘 飞, 许 杰, 白玉玺, 吕 剑. 髓芯减压联合牙髓干细胞治疗兔早期激素性股骨头坏死[J]. 中国组织工程研究, 2022, 26(7): 1074-1079. |
| [9] | 房晓磊, 冷 军, 张 晨, 刘会敏, 郭 文. 间充质干细胞不同移植途径治疗缺血性脑卒中疗效差异的系统评价[J]. 中国组织工程研究, 2022, 26(7): 1085-1092. |
| [10] | 郭 嘉, 丁琼桦, 刘 泽, 吕思懿, 周泉程, 高玉花, 白春雨. 间充质干细胞来源外泌体的生物学特性及免疫调控作用[J]. 中国组织工程研究, 2022, 26(7): 1093-1101. |
| [11] | 张璟琳, 冷 敏, 朱博恒, 汪 虹. 干细胞源外泌体促进糖尿病创面愈合的机制及应用[J]. 中国组织工程研究, 2022, 26(7): 1113-1118. |
| [12] | 黄晨玮, 费彦亢, 朱梦梅, 李鹏昊, 于 兵. 谷胱甘肽在干细胞“干性”及调控中的重要作用[J]. 中国组织工程研究, 2022, 26(7): 1119-1124. |
| [13] | 惠小珊, 白 京, 周思远, 王 阶, 张金生, 何庆勇, 孟培培. 中医药调控干细胞诱导分化的理论机制[J]. 中国组织工程研究, 2022, 26(7): 1125-1129. |
| [14] | 安维政, 何 萧, 任 帅, 刘建宇. 肌源干细胞在周围神经再生中的潜力[J]. 中国组织工程研究, 2022, 26(7): 1130-1136. |
| [15] | 范一鸣, 刘方煜, 张洪宇, 李 帅, 王岩松. 脊髓损伤后室管膜区内源性神经干细胞反应的系列问题[J]. 中国组织工程研究, 2022, 26(7): 1137-1142. |
组织工程是结合了生物学、材料学、工程学等为一体的多交叉性学科,致力于发展生物材料以修复、替代损伤组织,改善人体器官生理状态及提高其功能,并尽量实现外形与损伤前相一致的人性化需求[3]。
组织工程研究主要有3个要素:种子细胞、支架材料、细胞生长因子,其中种子细胞和支架材料是组织工程目前研究的重点内容。细胞是一切生物组织最基本的结构和功能单位。干细胞作为人体内一种有潜力的能够分化为其他类型细胞的特殊细胞,是生物工程广泛研究和利用的原材料。支架是用于支撑细胞成长为一个完整组织的框架材料,而人工设计制造的细胞外基质作为组织工程研究的支架材料,是组织工程研究的另一个重要组成部分,它为细胞的生长、黏附、迁移、增殖、分化和细胞间交流提供支持。
种子细胞与支架材料的相互作用贯穿组织工程的整个过程,设计出更符合种子细胞生存的基质微环境决定着组织工程的成败。因此,阐明基质微环境与细胞相互作用机制对组织工程具有重要的意义。力学信号是支架材料影响细胞的重要方式,能引导和协调组织内的细胞活动。目前已知的能够影响细胞活动的材料力学因素包括:支架材料的硬度、弹性、粗糙度和亲水性等,其中支架材料硬度对细胞行为的影响占据重要地位,且在国内外研究资料中也是相对最深入的。该文章拟阐释支架材料形成的微环境硬度对细胞行为影响的作用机制。通过细胞对材料力学信号的感知、转导以及后续引发的一系列细胞生理反应等,以利于在设计和制作支架材料过程中改善力学性能,为组织工程服务,提升组织工程的实践转化意义。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
1.2 纳入与排除标准
1.2.1 纳入标准 根据文章题目及摘要进行初步筛选,通过文献精读和泛读后设计提炼出与文章相关的研究原著、综述。
1.2.2 排除标准 研究目的及内容与此研究无关的文献;重复性研究。
1.3 数据的提取 共检索到文献562篇,英文文献470篇,中文文献92篇,排除与研究目的相关性差及内容陈旧、质量不高、重复文献401篇,纳入161篇符合标准的文献进行综述,探讨了细胞外基质各种力学信号,如材料界面硬度、拓扑结构和亲/疏水性等,从力学识别、力学-化学信号转导、信号通路级联、下游蛋白活化、转录启动到蛋白表达,对细胞的扩展、增殖、迁移、分化、交流和其他各种特殊生理性质进行调控。
近年研究发现,模拟不同组织微环境基体的弹性或刚度,脂肪干细胞可以朝血管、脂肪和成骨多个方向发展,源于中胚层的脂肪干细胞甚至可以作用于外胚层和内胚层。材料力学性质对细胞分化的影响显现出前所未有的光明前景。当组织损伤发生时,多用自体组织移植来治疗,可是这种方法仍牺牲了人体其他组织的健康,脂肪干细胞具有提取容易、来源广泛并能朝着多个谱系分化等优点,这意味着人们可以花费最小的代价来进行多种组织培养。因此,未来组织工程支架材料研究的热点可能将集中于调控干预多功能干细胞的分化及生理活动,进而不断模拟人体内的细胞微环境,实现培养细胞在结构与功能上与体内细胞的统一,最终通过培养达到临床所需的组织结构。在这一进程中,对细胞与材料界面力学性质交互作用的探究意义非凡。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程

文题释义:
细胞外基质力学微环境:组织工程进行细胞培养时,支架材料为细胞提供的生存环境称细胞外基质微环境,而此处的“力学”强调了材料界面的硬度、孔隙率、拓扑结构和亲/疏水等力学性质。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
目前,组织工程已在生命科学和医学领域展现出不可忽视的潜力和影响,成功取得临床转化的研究更是治愈或挽救了无数患者。支架材料的生物力学评价/制备方法、干细胞筛选、外泌体作用、生物活性因子-细胞-细胞外基质的相互作用、数字化/人工智能在组织工程中的交叉运用等日益成为组织工程未来研究的热点,而该综述正是立足于生物微环境、机械微环境和细胞与材料基质间的相互作用这3大创新点,以力学信号组成和细胞受影响的生理功能为分类依据,阐述了细胞外基质力学微环境与细胞的相互作用。经检索,尚未有文献从此角度出发综述各种力学信号对细胞各项生理活动的影响,由此侧面证明了文章的独特与创新性,以期对未来组织工程方面的研究及其分子生物学机制提供参考。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||