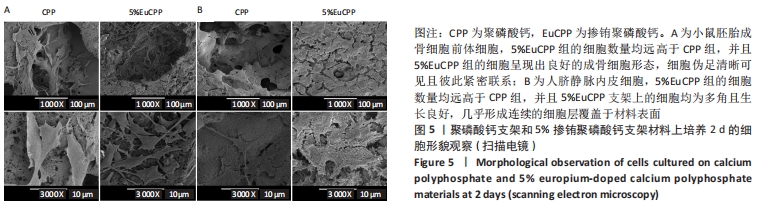
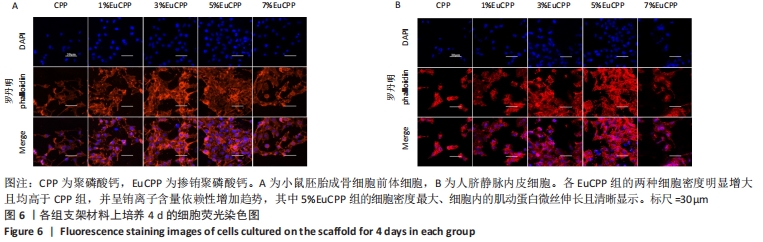
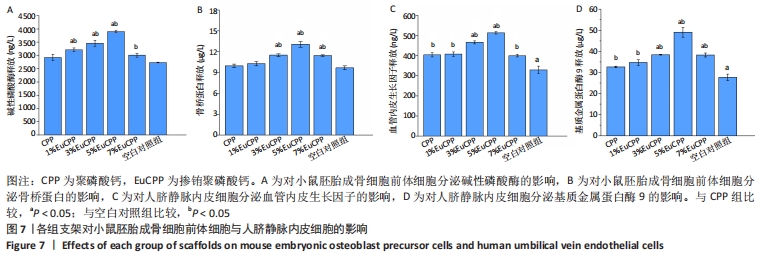
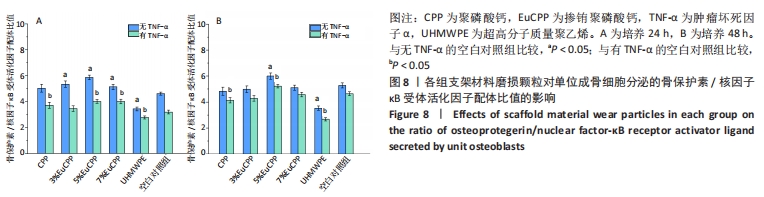
[1] ANTALYA H, JOHANNA B, RUSTOM LE, et al. Bone regeneration strategies: engineered scaffolds, bioactive molecules and stem cells Current stage and future perspectives. Biomaterials. 2018;180:143-162.
[2] HENCH LL, THOMPSON I. Twenty-first century challenges for biomaterials. J R Soc Interface. 2010;7(Suppl_4):379-391.
[3] PILLIAR RM, FILIAGGI MJ, WELLS JD, et al. Porous calcium polyphosphate scaffolds for bone substitute applications — in vitro characterization. Biomaterials. 2001;22(9):963-972.
[4] YOU C, LEE MH, LEE HJ, et al. The effect of macro/micro combination pore structure of biphasic calcium phosphate scaffold on bioactivity. Ceram Int. 2017;43(4):3540-3546.
[5] KIM TG, SHIN H, DONG WL. Biomimetic Scaffolds for Tissue Engineering. Adv Funct Mater. 2012;22(12):2446-2468.
[6] WU C, CHEN Z, YI D, et al. Multidirectional effects of Sr-, Mg-, and Si-containing bioceramic coatings with high bonding strength on inflammation, osteoclastogenesis, and osteogenesis. ACS Appl Mater Interfaces. 2014;6(6):4264-4276.
[7] GUO B, LEI B, LI P, et al. Functionalized scaffolds to enhance tissue regeneration. Regen Biomater. 2015;2(1):47-57.
[8] RATNAYAKE JTB, GOULD ML, SHAVANDI A, et al. Development and characterization of a xenograft material from New Zealand sourced bovine cancellous bone. J Biomed Mater Res B Appl Biomater. 2017; 105(5):1054-1062.
[9] HETTICH G, SCHIERJOTT RA, EPPLE M, et al. Calcium Phosphate Bone Graft Substitutes with High Mechanical Load Capacity and High Degree of Interconnecting Porosity. Materials (Basel). 2019;12(21):3471.
[10] BOSE S, FIELDING G, TARAFDER S, et al. Understanding of dopant-induced osteogenesis and angiogenesis in calcium phosphate ceramics. Trends Biotechnol. 2013;31(10):594-605.
[11] WANG X, GU Z, JIANG B, et al. Surface modification of strontium-doped porous bioactive ceramic scaffolds via poly(DOPA) coating and immobilizing silk fibroin for excellent angiogenic and osteogenic properties. Biomater Sci. 2016;4(4):678-688.
[12] VASCONCELOS DM, SANTOS SG, LAMGHARI M, et al. The two faces of metal ions: From implants rejection to tissue repair/regeneration. Biomaterials. 2016;84:262-275.
[13] LI Q, CHEN H, HUANG X, et al. Effects of 12 metal ions on iron regulatory protein 1 (IRP-1) and hypoxia-inducible factor-1 alpha (HIF-1α) and HIF-regulated genes. Toxicol Appl Pharmacol. 2006;213(3): 245-255.
[14] STEGEN S, DEPREZ S, EELEN G, et al. Adequate hypoxia inducible factor 1α signaling is indispensable for bone regeneration. Bone. 2016; 87:176-186.
[15] QIU K, ZHAO XJ, WAN CX, et al. Effect of strontium ions on the growth of ROS17/2.8 cells on porous calcium polyphosphate scaffolds. Biomaterials. 2006;27(8):1277-1286.
[16] WANG X, WANG Y, LI L, et al. Stimulations of strontium-doped calcium polyphosphate for bone tissue engineering to protein secretion and mRNA expression of the angiogenic growth factors from endothelial cells in vitro. Ceram Int. 2014;40(5):6999-7005.
[17] PATRA CR, BHATTACHARYA R, PATRA S, et al. Pro-angiogenic Properties of Europium(III) Hydroxide Nanorods. Adv Mater. 2008;20(4):753-756.
[18] PATRA CR, ABDEL MONEIM SS, WANG E, et al. In vivo toxicity studies of europium hydroxide nanorods in mice. Toxicol Appl Pharmacol. 2009; 240(1):88-98.
[19] BARTA CA, SACHS-BARRABLE K, JIA J, et al. Lanthanide containing compounds for therapeutic care in bone resorption disorders. Dalton Trans. 2007;(43):5019-5030.
[20] ZHONG M, ZHANG X, SHI X, et al. Halofuginone inhibits LPS-induced attachment of monocytes to HUVECs. Int Immunopharmacol. 2020; 87:106753.
[21] 刘广建,岳文贞,陈荣春.UHMWPE用于生物工程的摩擦磨损研究[J].工程塑料应用,2002,30(1):36-38.
[22] FANG HW, HO YC, YANG CB, et al. Preparation of UHMWPE particles and establishment of inverted macrophage cell model to investigate wear particles induced bioactivites. J Biochem Biophys Methods. 2006; 68(3):175-187.
[23] BALOGH E, PARAGH G, JENEY V. Influence of Iron on Bone Homeostasis. Pharmaceuticals (Basel). 2018;11(4):107.
[24] GARCÍA-GARETA E, COATHUP MJ, BLUNN GW. Osteoinduction of bone grafting materials for bone repair and regeneration. Bone. 2015;81: 112-121.
[25] SOUS M, BAREILLE R, ROUAIS F, et al. Cellular biocompatibility and resistance to compression of macroporous beta-tricalcium phosphate ceramics. Biomaterials. 1998;19(23):2147-2153.
[26] MEI L, HONG F, LI W, et al. Size-dependent microstructure and europium site preference influence fluorescent properties of Eu3+-doped Ca10(PO4)6(OH)2 nanocrystal. J Lumin. 2008;128(3):428-436.
[27] MIAO G, CHEN X, MAO C, et al. Synthesis and characterization of europium-containing luminescent bioactive glasses and evaluation of in vitro bioactivity and cytotoxicity. J Solgel Sci Technol. 2014;69(2): 250-259.
[28] SONG W, TIAN M, CHEN F, et al. The study on the degradation and mineralization mechanism of ion-doped calcium polyphosphate in vitro. J Biomed Mater Res B Appl Biomater. 2009;89(2):430-438.
[29] ZHAO R, XIE P, ZHANG K, et al. Selective effect of hydroxyapatite nanoparticles on osteoporotic and healthy bone formation correlates with intracellular calcium homeostasis regulation. Acta Biomater. 2017; 59:338-350.
[30] JENSEN T, BAAS J, DOLATHSHAHI-PIROUZ A, et al. Osteopontin functionalization of hydroxyapatite nanoparticles in a PDLLA matrix promotes bone formation. J Biomed Mater Res A. 2011;99(1):94-101.
[31] LIU F, ZHANG X, YU X, et al. In vitro study in stimulating the secretion of angiogenic growth factors of strontium-doped calcium polyphosphate for bone tissue engineering. J Mater Sci Mater Med. 2011;22(3):683-692.
[32] HEGDE PS, WALLIN JJ, MANCAO C. Predictive markers of anti-VEGF and emerging role of angiogenesis inhibitors as immunotherapeutics. Semin Cancer Biol. 2018;52(Pt 2):117-124.
[33] ADYA R, TAN BK, PUNN A, et al. Visfatin induces human endothelial VEGF and MMP-2/9 production via MAPK and PI3K/Akt signalling pathways: novel insights into visfatin-induced angiogenesis. Cardiovasc Res. 2008;78(2):356-365.
[34] WU C, XIA L, HAN P, et al. Europium-Containing Mesoporous Bioactive Glass Scaffolds for Stimulating in Vitro and in Vivo Osteogenesis. ACS Appl Mater Interfaces. 2016;8(18):11342-11354.
[35] HOLLBORN M, STATHOPOULOS C, STEFFEN A, et al. Positive feedback regulation between MMP-9 and VEGF in human RPE cells. Invest Ophthalmol Vis Sci. 2007;48(9):4360-4367.
[36] PARK JH, LEE NK, LEE SY. Current Understanding of RANK Signaling in Osteoclast Differentiation and Maturation. Mol Cells. 2017;40(10):706-713.
[37] CHEN X, WANG Z, DUAN N, et al. Osteoblast-osteoclast interactions. Connect Tissue Res. 2017;59(2):99-107.
[38] KENKRE JS, BASSETT J. The bone remodelling cycle. Ann Clin Biochem. 2018;55(3):308-327.
[39] BOYCE BF, XING L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys. 2008;473(2):139-146.
[40] 陈昱铭,曾晖,熊奡,等.TNF-α对人滑膜细胞RANKL、OPGmRNA表达的影响[J].中国现代医学杂志,2009,19(9):1301-1303,1307.
[41] CHILDS LM, GOATER JJ, O’KEEFE RJ, et al. Efficacy of etanercept for wear debris-induced osteolysis. J Bone Miner Res. 2001;16(2):338-347.
[42] BLADEN CL, TZU-YIN L, FISHER J, et al. In vitro analysis of the cytotoxic and anti-inflammatory effects of antioxidant compounds used as additives in ultra high-molecular weight polyethylene in total joint replacement components. J Biomed Mater Res B Appl Biomater. 2013; 101(3):407-413.
[43] HACCHOU Y, UEMATSU T, UEDA O, et al. Inorganic polyphosphate: a possible stimulant of bone formation. J Dent Res. 2007;86(9):893-897.
|