中国组织工程研究 ›› 2019, Vol. 23 ›› Issue (2): 196-203.doi: 10.3969/j.issn.2095-4344.1507
• 药物控释材料 drug delivery materials • 上一篇 下一篇
可注射性乙二醇壳聚糖/双醛功能化聚乙二醇水凝胶的细胞相容性
荆晓光1,2,刘舒云2,郭维民2,李 旭2,吕 奥2,刘士臣1,孟昊业2,陈明学2,张学亮2,张增增1,2,刘雪剑1,2,高 超1,2,王泽浩2,张 彬2,沈 师2,陶 磊3,杨建华1,4,郭全义2
- 1佳木斯大学,黑龙江省佳木斯市 154007;2解放军总医院骨科研究所,北京市 100853;3清华大学化学系,北京市 100084;4深圳市龙岗区人民医院,广东省深圳市 518172
Cytocompatibility of injectable glycol chitosan/dibenzaldehyde-terminated poly-ethyleneglycol hydrogel
Jing Xiaoguang1, 2, Liu Shuyun2, Guo Weimin2, Li Xu2, Lü Ao2, Liu Shichen1, Meng Haoye2, Chen Mingxue2, Zhang Xueliang2, Zhang Zengzeng1, 2, Liu Xuejian1, 2, Gao Chao1, 2, Wang Zehao2, Zhang Bin2, Shen Shi2, Tao Lei3, Yang Jianhua1, 4, Guo Quanyi2
- 1Jiamusi University, Jiamusi 154007, Heilongjiang Province, China; 2Institute of Orthopedics, Chinese PLA General Hospital, Beijing 100853, China; 3Department of Chemistry, Tsinghua University, Beijing 100084, China; 4Longguang People’s Hospital, Shenzhen 518172, Guangdong Province, China
摘要:
文章快速阅读:
.jpg)
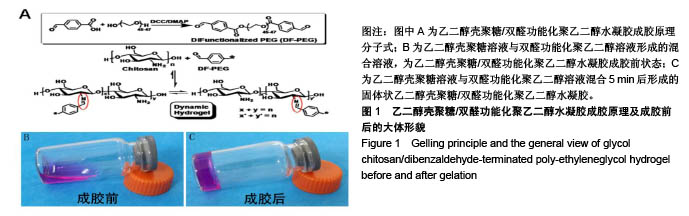
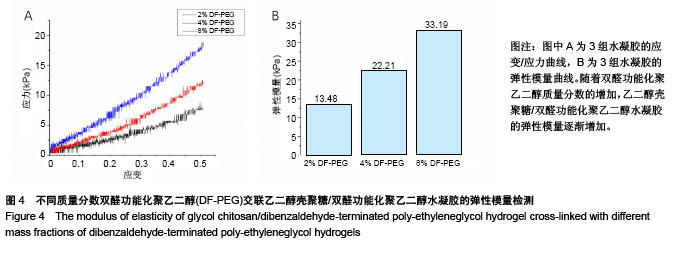
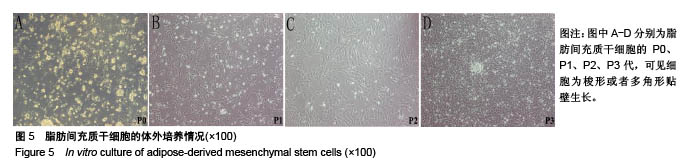
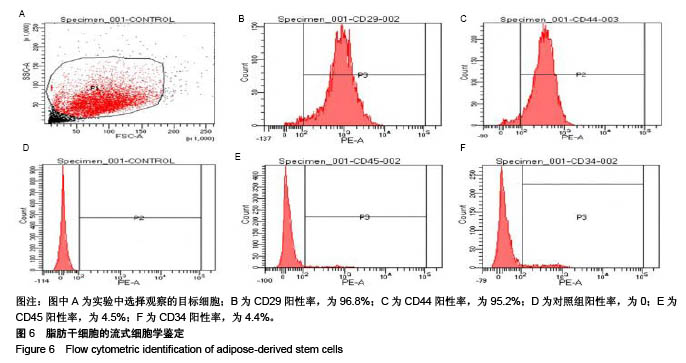
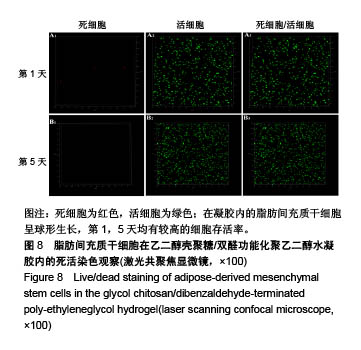
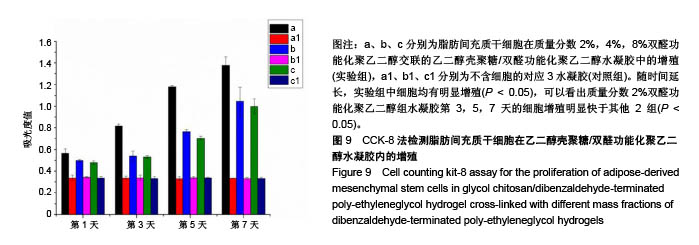
背景:课题组前期研究自主研发了可注射性乙二醇壳聚糖/双醛功能化聚乙二醇水凝胶(glycol chitosan/ dibenzaldehyde-terminated poly-ethyleneglycol,GCS/DF-PEG),研究显示其具有较好的可注射性和自愈性。 目的:进一步检测可注射性GCS/DF-PEG的物理学性质及细胞相容性。 方法:将质量分数1.5%的乙二醇壳聚糖溶液与质量分数分别为2%,4%,8%的双醛功能化聚乙二醇溶液等体积混合,制备3组可注射性GCS/DF-PEG水凝胶,检测其弹性模量。将3组可注射性水凝胶分别浸入PBS中4周,检测凝胶的体外降解。提取第2代SD乳鼠脂肪间充质干细胞,实验组加入可注射性GCS/DF-PEG水凝胶浸提液,对照组常规培养,MTT法检测细胞增殖。将质量分数3%的乙二醇壳聚糖溶液与第2代SD乳鼠脂肪间充质干细胞混合,再与质量分数为4%的双醛功能化聚乙二醇溶液混合,培养第1,5 天,采用死活染色法检测细胞在水凝胶内死活状态。将质量分数3%的乙二醇壳聚糖溶液与第2代SD乳鼠脂肪间充质干细胞混合,再与质量分数分别为2%,4%,8%的双醛功能化聚乙二醇溶液混合,MTT法检测细胞增殖。 结果与结论:①质量分数分别为2%,4%,8%双醛功能化聚乙二醇组水凝胶的弹性模量分别为13.48,22.21,33.19 kPa;②随时间的延长,3组水凝胶的降解率均逐渐增加,质量分数2%双醛功能化聚乙二醇组水凝胶的降解速率明显快于其他两组;③培养7 d内,实验组细胞增殖与对照组比较无差异;④死活染色显示,脂肪间充质干细胞在水凝胶内呈球形,存活率在90%以上,且随时间延长细胞数量明显增多;⑤随着时间的延长,脂肪间充质干细胞在含不同质量分数双醛功能化聚乙二醇GCS/DF-PEG水凝胶内的数量逐渐增多,且含质量分数2%双醛功能化聚乙二醇组水凝胶内的细胞增殖快于含质量分数4%,8%双醛功能化聚乙二醇组(P < 0.05);⑥结果表明,可注射性GCS/DF-PEG水凝胶的细胞相容性良好,力学强度及降解具有可调性,有望成为软骨组织工程中干细胞移植的良好载体。
ORCID: 0000-0002-6424-3242(荆晓光)
中图分类号:



.jpg)