Chinese Journal of Tissue Engineering Research ›› 2022, Vol. 26 ›› Issue (28): 4562-4568.doi: 10.12307/2022.314
Previous Articles Next Articles
Application of biomaterial scaffolds in the treatment of spinal cord injury
Zhao Xingchang1, 2, Song Shiqiang1, He Feng1, Tang Yujin3, Liu Jia3
- 1Youjiang Medical University for Nationalities, Baise 533000, Guangxi Zhuang Autonomous Region, China; 2Handan Hospital of Traditional Chinese Medicine, Handan 056000, Hebei Province, China; 3Affilialted Hospital of Youjiang Medical University for Nationalities, Baise 533000, Guangxi Zhuang Autonomous Region, China
-
Received:2021-02-05Accepted:2021-04-10Online:2022-10-08Published:2022-03-24 -
Contact:Liu Jia, MD, Professor, Chief physician, Master’s supervisor, Affilialted Hospital of Youjiang Medical University for Nationalities, Baise 533000, Guangxi Zhuang Autonomous Region, China -
About author:Zhao Xingchang, Master, Attending physician, Youjiang Medical University for Nationalities, Baise 533000, Guangxi Zhuang Autonomous Region, China; Handan Hospital of Traditional Chinese Medicine, Handan 056000, Hebei Province, China -
Supported by:the National Natural Science Foundation of China, No. 82071361, No. 81560213 (to LJ); Science and Technology Project of Guangxi Zhuang Autonomous Region, No. 2018GXNSFAA138074 (to LJ)
CLC Number:
Cite this article
Zhao Xingchang, Song Shiqiang, He Feng, Tang Yujin, Liu Jia. Application of biomaterial scaffolds in the treatment of spinal cord injury[J]. Chinese Journal of Tissue Engineering Research, 2022, 26(28): 4562-4568.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
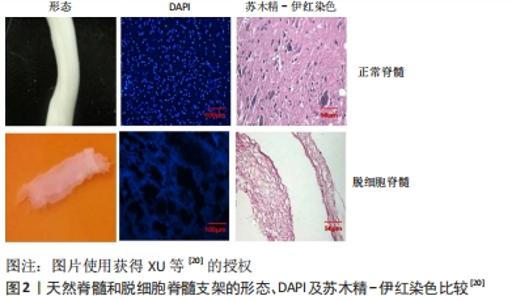
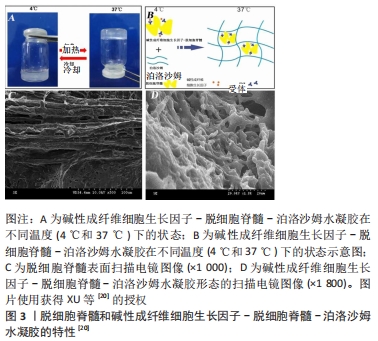
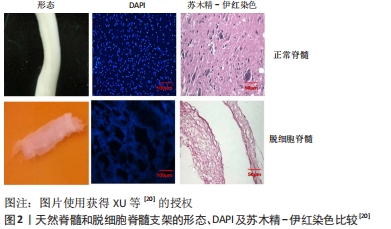
“天然生物材料”是相对于人工合成生物材料而言,指天然形成的可直接使用的材料,目前主要应用的有生物组织、蛋白及天然纤维等。天然生物材料应具有相容性好[11]、抗原排斥小、降解产物毒性低、可提供神经细胞生长的环境而诱导轴突再生、促进神经细胞黏附及增殖等优点[12-13];但也存在作为支架时支撑强度不够、吸水后溶胀而致三维立体结构塌陷、降解时间与神经再生所需时间不匹配等问题。生物组织材料主要有脱细胞脊髓支架、生物材料的蛋白,天然纤维主要有胶原、纤维蛋白、透明质酸、壳聚糖等,这些材料同样具有相容性好、无毒、可降解等优势,但缺乏脱细胞脊髓支架多种细胞外基质的成分。现将各种材料分述如下。 2.1.1 脱细胞脊髓及其复合支架的应用 脱细胞脊髓支架是将从实验动物体内获取的脊髓,经化学脱细胞、物理洗涤、超声、冰融及混合方法处理去除脊髓神经细胞,经冰冻干燥所得的多孔脱细胞支架。脱细胞脊髓支架在修复脊髓损伤中有其独特之处:脱细胞后保留了脊髓的细胞外基质天然成分(主要包括胶原蛋白、非胶原蛋白、弹性蛋白、蛋白聚糖与氨基聚糖等),不仅可以支持脊髓神经再生,而且还含有大量信号分子,积极参与调控神经细胞的生长、迁移和代谢再生[14];支架来源于脊髓,因此与脊髓组织三维结构相似,能为神经轴突生长提供通道而定向生长,避免了细胞成团或无序生长,成为载入干细胞、药物、神经营养因子良好的载体[15];支架为天然生物材料,组织相容性好、抗原排斥小、降解性好且降解产物无毒性反应等,单独将生物材料支架植入脊髓损伤模型虽然取得了一定的效果,但脊髓组织再生的效果有待提高,所以目前通常将支架作为负载干细胞、药物、神经营养因子等因素的载体应用到脊髓损伤修复中,以期提高神经细胞的再生率和定向生长。 WANG等[16]发现,脱细胞脊髓支架联合骨髓间充质干细胞可通过控制细胞凋亡和炎症来促进脊髓损伤的修复,联合支架较单独应用脱细胞脊髓支架运动功能恢复更好。BAN等[17]用化学萃取法制备了脱细胞脊髓支架,支架具有良好的三维网状多孔结构,与神经元体外共培养显示孔道结构内充满神经元且细胞存活率为(97.53±1.52)%,表明脱细胞脊髓支架是神经元再生的良好基础,并可促进轴突再生。LIU等[18]将脱细胞脊髓支架与抑癌基因PTEN抑制剂bpV(pic)相结合,发现脱细胞脊髓联合BpV(pic)可减少神经元丢失,靶向PTEN使巨噬细胞向M2极化,确定了PTEN对巨噬细胞的极化和功能恢复至关重要。脱细胞脊髓/神经营养蛋白3复合支架可很好地促进大鼠骨髓间充质干细胞的黏附、增殖和分化并促进运动功能恢复[19]。脱细胞脊髓-碱性成纤维细胞生长因子-泊洛沙姆复合支架水凝胶,脱细胞脊髓支架提高了碱性成纤维细胞生长因子的稳定性并使碱性成纤维细胞生长因子释放减慢,促进PC12细胞的增殖,抑制胶质瘢痕,但未对碱性成纤维细胞生长因子释放及作用时间进一步研究[20],见图2,3。脱细胞脊髓支架联合血管内皮生长因子可促进脊髓损伤后的血管生成和血管重构,但在动物模型中脊髓功能的改善是有限的[21]。"

因此,脱细胞脊髓支架可单独用于脊髓损伤,三维多孔结构及保留的脊髓细胞外基质天然成分可以支持脊髓神经再生,参与调控神经细胞的生长、迁移。但大部分研究将脱细胞脊髓支架作为骨髓间充质干细胞、血管内皮生长因子、碱性成纤维细胞生长因子等神经生长因子的载体,神经轴突再生和神经干细胞分化较单独应用脱细胞脊髓支架效果好。同时也可将脱细胞脊髓支架与促进神经再生、抑制胶质细胞成长的药物结合制作成复合支架,植入脊髓损伤模型中,术后神经再生、动物肢体功能恢复也能取得较好的效果。 2.1.2 天然纤维在脊髓损伤修复中的应用 将天然纤维制作成管状或通过3D打印成具有高孔隙、三维多孔结构的支架可被用作脊髓损伤修复材料,此类支架同样具有组织相容性好、可生物降解、低毒、三维立体等优点,将其植入脊髓损伤模型中可促进神经干细胞的黏附、增殖、分化。目前应用较广的材料有壳聚糖、透明质酸、胶原、纤维蛋白等。 壳聚糖基及其复合材料支架:壳聚糖是甲壳类动物外骨骼、真菌细胞和鱿鱼中提取的天然多糖,制成的材料具有独特的组织相容性、生物降解性和无毒性等。ZHAO等[22]利用壳聚糖制备成具有多孔结构的复合水凝胶支架,支架对人源骨髓间充质干细胞有较好的吸附作用,在干细胞诱导后仍保持了较高的保真性和分化潜能,但由于未进行动物实验,不能明确对脊髓功能恢复的作用。壳聚糖水凝胶复合支架可通过调节局部免疫和恢复血管功能促进再生轴突的髓鞘化和鞘膜化,促进神经功能恢复[23]。含有周围神经的壳聚糖支架能诱导大量许旺细胞长入,并有良好的组织相容性,植入脊髓损伤模型后14周未见明显炎症反应[24]。Yao等[25]将壳聚糖支架、海藻酸钠支架和壳聚糖-海藻酸钠支架修复脊髓损伤做了对比,发现3种支架均有良好的生物相容性和降解性,但海藻酸钠支架降解速度快,不能长期支撑和桥接作用。壳聚糖支架具有更好的生物相容性,能够促进神经纤维的再生,防止瘢痕组织的形成,更适合脊髓损伤的修复。 因此,壳聚糖作为生物材料,具有良好的组织相容性、三维多孔结构,但机械强度、降解速率等研究较少,如不能在满足神经再生的条件下降解,或降解时间较长,则会影响神经细胞生长并有可能激活多种炎症反应。 透明质酸基及其复合支架/修复材料:透明质酸的分子结构中含有大量的羧基和羟基,在水溶液中形成分子内和分子间的氢键,使其具有强大的保水作用;作为细胞间基质的主要成分,直接调控细胞内外电解质的平衡,发挥物理和分子信息的过滤器作用。刘东等[26]将Nogo-66受体抗体和多聚左旋赖氨酸接枝到透明质酸水凝胶支架上,然后与携带β-神经生长因子腺病毒转染的内皮祖细胞体外共培养,结果显示内皮祖细胞在支架上的生长明显优于培养板中的生长状况且具有良好的生物相容性。 透明质酸在细胞外基质中广泛存在,具有易得性,且分子结构与生物体内组织一致,不会引起机体免疫反应;但透明质酸存在易溶于水、吸收降解较快等缺点,不能很好满足修复脊髓损伤的要求。因此需要对透明质酸进行交联或加入药物后再交联,可通过光交联或化学交联,交联后形所的复合生物材料能解决溶于水后快速降解的缺点,被广泛应用于脊髓损伤修复领域。 纤维蛋白基及其复合支架/修复材料:纤维蛋白是一种蛋白质多聚体,主要来源于血浆蛋白,因此具有明显的血液和组织相容性,无毒副作用;可交织成多孔的网状结构,为装载种子细胞和药物、各种营养因提供了载体,并且所含蛋白可促进细胞的黏附、增殖,被广泛应用在修复脊髓损伤的研究中[27-28]。利用静电纺丝技术制作的纤维蛋白水凝胶支架可诱导细胞定向侵入、血管系统重建和轴突再生,进而促进轴突定向再生和运动功能恢复[29]。纤维蛋白支架与脂肪间充质干细胞结合可减少星形胶质细胞激活,改善微环境并促进神经再生[27]。JALALI MONFARED等[30]发现用纤维蛋白水凝胶包裹人类子宫内膜干细胞,可将人子宫内膜干细胞分化为少突胶质细胞祖细胞而修复神经损伤。纤维蛋白具有较好的组织相容性且作为载体包裹药物及神经生长因子能缓慢释放。因此对中枢神经修复大部分应用其包裹作用,也可与其他生物材料作制成复合材料支架促进轴突再生,减少胶质瘢痕形成,从而修复脊髓损伤,运动功能明显恢复。 胶原蛋白基及其复合支架/修复材料:胶原蛋白是细胞外基质的一种重点成分,3条多肽链组成的特殊结构使其具有更高的位伸强度,使生长因子能与细胞表面结合位点结合进一步促进神经的修复[31]。WANG等[32]发现胶原-血管内皮生长因子复合支架可控制性干预改善微环境,促进新生血管形成,诱导轴突生长,促进神经功能恢复。胶原蛋白结合碱性成纤维细胞生长因子复合支架可通过抑制损伤区瘢痕形成、作为干细胞迁移的载体而修复损伤[33]。胶原蛋白联合人脐血间充质干细胞复合支架能促进神经再生和再髓鞘化,阻断星形胶质细胞区域外的生长,为神经再生提供有利的微环境[34]。FAN等[35]将胶原支架改良成线样,负载表皮细胞生长因子及药物形成的复合支架能中和髓鞘抑制分子,很好地引导神经再生和抑制瘢痕形成,进而恢复神经功能。将胶原蛋白制作成支架并负载促神经生长因子可促进神经再生和抑制胶质瘢痕形成,但存在植入脊髓损伤区后易降解、支架塌陷等缺点,因此,胶原蛋白如能在脊髓损伤修复方面取得更好的效果,需要对其加工或加入互补的其他生物材料,弥补易降解、支撑强度不够的缺点。 2.2 人工合成生物材料支架及应用 相对于天然支架而言,合成材料具有原料易得、理化性质、空间结构、强度、降解率可调等优势,已在科研中得到广泛应用。人工合成材料支架应用在脊髓损伤修复中应该具备如下特征:①良好的组织相容性:即与接触的周围组织无明显抗原排斥反应,只有相容性好,才能提供神经干细胞分化、繁殖所需的微环境,使脊髓功能恢复成为可能。如相容性不佳则炎症反应明显,自噬系统启动,神经干细胞不可能再生。②强度与降解速率的完美匹配:支架应有一定的强度来起到“桥梁”的作用,使神经细胞定向生长,同时应与降解速率匹配,如降解速度过快,不能起到支撑效果;如降解速度过慢则影响脊髓组织的再构。③具备三维立体、高孔率结构:只有具备多孔、三维立体结构,才能为神经细胞更好地黏附、分化、增殖、再生及顺利向两端爬行等提供足够的空间。目前应用的合成材料主要有聚己内酯、聚乳酸、聚乳酸-聚羟基乙酸、聚乙二醇、水凝胶及各种材料的混合等。"

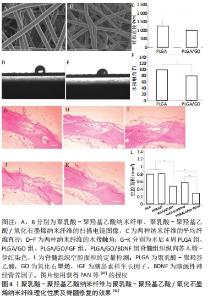
2.2.1 聚己内酯基及其复合支架/修复材料 聚己内酯具有良好的生物相容性、生物降解性,可支撑细胞生长。装载许旺细胞和诱导多能干细胞的聚己内酯支架,可诱导多能干细胞在体外转化为神经干细胞,减少了病变的体积且促进了功能恢复[36]。聚唾液酸/甲基泼尼龙/聚己内酯复合支架可抑制小胶质细胞/巨噬细胞激活,降低肿瘤坏死因子α和白细胞介素6的释放及凋亡相关Caspase-3蛋白的表达,进而促进脊髓损伤后轴突生长及功能的恢复[37]。将人子宫内膜干细胞与人许旺细胞接种到聚己内酯/明胶支架上可诱导人子宫内膜干细胞转化为神经干细胞,修复脊髓损伤[38]。电纺丝聚己内酯支架能诱导人子宫内膜干细胞的黏附和生长,减少脊髓损伤后空洞的形成,促进脊髓恢复[39]。聚己内酯多与其他生物材料通过各种工艺制作成多孔三维结构、生物相容物好、可作为神经细胞黏附和生长基础的复合支架,并负载细胞或神经生长因子,较单独应用聚己内酯修复脊髓损伤的效果好。 2.2.2 聚乳酸、聚乳酸-聚羟基乙酸及其复合支架/材料 聚乳酸是主要以乳酸为原料聚合得到的一种新型生物材料,同样具有良好的生物相容性和降解性。聚乳酸/聚吡咯纳米纤维复合支架负载骨髓间充质干细胞可抑制胶质瘢痕、促进轴突再生和弥合病变的间隙,并恢复神经电传导[40]。SANTHOSH等[41]通过制备聚乳酸-聚羟基乙酸微粒,将神经调节素1封装到聚乳酸-羟基乙酸微颗粒中,通过控制聚乳酸-聚羟基乙酸颗粒的孔隙率和大小来调控神经调节素1的释放速率和时间,促进少突胶质细胞分化,减轻脊髓损伤后的神经炎症和神经胶质瘢痕,从而促进神经功能的恢复。氧化石墨烯/聚乳酸-聚羟基乙酸电纺纳米纤维支架负载生长因子胰岛素样生长因子1和脑源性神经营养因子,能保护神经干细胞免受H2O2诱导的氧化应激,还能增强神经干细胞的增殖和神经分化;增加损伤部位的神经元数量,并减少空洞的形成[42],见图4。"
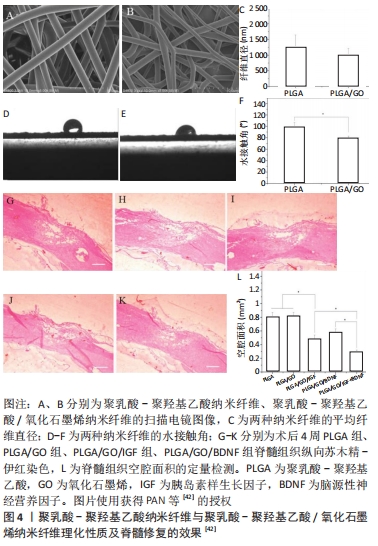
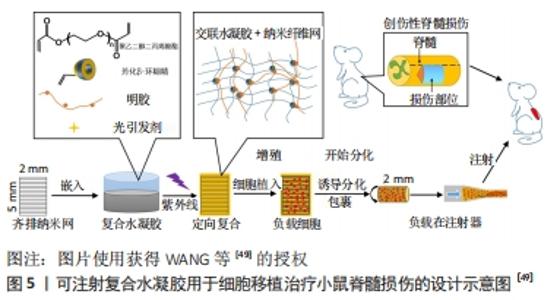
含有脑源性神经营养因子和血管内皮生长因子的聚乳酸-聚羟基乙酸微球与结合抗Nogo受体抗体(antiNgR)混合,可以改善局部微环境,抑制炎症和胶质增生,特别是在植入物内部和周围发现了大量的新生血管和再生神经纤维,并且表现出更好的运动恢复[43]。WANG等[44]将嗅鞘细胞接种到聚乳酸-聚羟基乙酸支架,发现局部促进了细胞分化和抑制星形胶质细胞来增强再髓鞘化形成,可能得益于微环境的改善。以上研究发现,聚乳酸本身具有良好的组织相容性及降解性对神经元再生有促进作用。但大部分研究将聚乳酸与其他生物材料制备成复合支架并负载神经生长因子,能在改善修复微环境基础上促进轴突再生,抑制胶质瘢痕形成,比单独应用聚乳酸支架的效果好。 2.2.3 聚乙二醇及其复合支架/材料 聚乙二醇具有良好的水溶性,能与材料表面吸附,可制备成微凝胶、薄膜、控释等复合材料。聚乙二醇微凝胶支架能用于人骨髓间充质干细胞的传递并促进其分泌潜力,从而修复神经损伤[45]。聚乙二醇可介导细胞膜再封闭来修复或逆转损伤后细胞持续损伤,不仅可以减少急性坏死,还可以减少细胞凋亡;聚乙二醇还直接保护线粒体功能,这就为聚乙二醇联合药物治疗脊髓损伤提供了支撑[46]。KIM等[47]利用聚乙二醇具有修复脊髓损伤后神经细胞膜的能力,将聚乙二醇直接滴入颈髓损伤模型内,术后颈髓损伤仅1 h就可出现神经生理运动传导;同时也可用于较长时间脊髓损伤的病例,瘢痕切除并将聚乙二醇植入所形成的空腔,可诱导组织恢复、轴突再生、髓鞘形成和功能改善。聚乙二醇对神经修复的主要优点为可诱导细胞膜再封闭并保护线粒体,逆转细胞持续受损,减少细胞凋亡。 2.3 水凝胶及其复合支架/材料的应用 水凝胶可具有三维多孔网状结构,为神经干细胞分化、再生、繁殖提供微环境;同时具有较好的组织相容性,可单独应用或作为载体而修复损伤的脊髓。水凝胶可分为天然生物材料水凝胶、人工合成类水凝胶、经修饰负载生物活性物质的水凝胶和自组装肽水凝胶等4类。 各类水凝胶有各自不同的特点:①天然生物材料水凝胶:如胶原-壳聚糖水凝胶支架,利用壳聚糖较好的延展性弥补胶原易分解、支撑力不够等问题,促进神经元再生[48];透明质酸水凝胶能促进神经干细胞的黏附,为神经元提提供良好的修复微环境[26]。②人工合成类水凝胶:WANG等[49]制备了可注射聚乙二醇(二醇)二丙烯酸酯纳米增强形状记忆水凝胶系统,极大地提高了体外人胚胎干细胞的活力和它们向神经干细胞的分化,促进小鼠脊髓损伤修复再生和运动功能恢复,见图5。"

③经修饰负载药物及生长因子的水凝胶:HAN等[50]制备可注射含牛熊去氧胆酸的水凝胶,抑制炎症反应、抗凋亡促进神经功能恢复;赵宣淇等[51]制备神经营养因子3基因修饰的骨髓间充质干细胞、水凝胶复合支架,能使脊髓损伤区域星形胶质细胞减少,胶质瘢痕减少而促进神经功能恢复;NAZEMI等[52]制备米诺环素和紫杉醇/水凝胶可注射双药物供给系统,可减少小胶质细胞/巨噬细胞浸润、降低凋亡因子caspase-3的表达、减少脊髓损伤区炎症反应;ALBASHARI等[53]制备了牙髓干细胞/热敏水凝胶包裹成纤维细胞生长因子,促进神经元轴突再生。④自组装技术:HU等[54]制备热敏肝素-泊洛沙默水凝并包裹成纤维细胞生长因子和神经生长因子,通过激活磷脂酰肌醇3激酶和蛋白激酶B(PI3K/Akt)及丝裂原活化蛋白激酶/细胞外信号调节激酶(MAPK/ERK)信号通路实现治疗脊髓损伤。水凝胶可作为支架负载神经营养因子、药物,改善微环境用于脊髓损伤的修复,促进神经增殖、分化,改善运动功能,为脊髓神经的修复提供了支撑。"

| [1] AHUJA CS, NORI S, TETREAULT L, et al. Traumatic Spinal Cord Injury-Repair and Regeneration. Neurosurgery. 2017;80(3s):S9-s22. [2] WU J, LIPINSKI MM. Autophagy in Neurotrauma: Good, Bad, or Dysregulated. Cells. 2019;8(7):693. [3] FAN B, WEI Z, YAO X, et al. Microenvironment Imbalance of Spinal Cord Injury. Cell Transplant. 2018;27(6):853-866. [4] COFANO F, BOIDO M, MONTICELLI M, et al. Mesenchymal Stem Cells for Spinal Cord Injury: Current Options, Limitations, and Future of Cell Therapy. Int J Mol Sci. 2019;20(11):2698. [5] 刘佳.脊髓脱细胞支架复合人脐血间充质干细胞促进大鼠脊髓长节段缺损轴突长入再生及功能恢复[D].广州:南方医科大学,2013. [6] KATOH H, YOKOTA K, FEHLINGS MG. Regeneration of Spinal Cord Connectivity Through Stem Cell Transplantation and Biomaterial Scaffolds. Front Cell Neurosci. 2019;13:248. [7] LI X, LIU D, XIAO Z, et al. Scaffold-facilitated locomotor improvement post complete spinal cord injury: Motor axon regeneration versus endogenous neuronal relay formation. Biomaterials. 2019;197:20-31. [8] DEAL DN, GRIFFIN JW, HOGAN MV. Nerve conduits for nerve repair or reconstruction. J Am Acad Orthop Surg. 2012;20(2):63-68. [9] CHEN JC, LI LM, GAO JQ. Biomaterials for local drug delivery in central nervous system. Int J Pharm. 2019;560:92-100. [10] ZHANG J, YUN S, BI J, et al. Enhanced multi-lineage differentiation of human mesenchymal stem/stromal cells within poly(N-isopropy lacrylamide-acrylic acid) microgel-formed three-dimensional constructs. J Mater Chem B Mater Biol Med. 2018;6(12):1799-1814. [11] OLIVEIRA MB, RIBEIRO MP, MIGUEL SP, et al. In vivo high-content evaluation of three-dimensional scaffolds biocompatibility. Tissue Eng Part C Methods. 2014;20(11):851-864. [12] ZHOU HL, ZHANG XJ, ZHANG MY, et al. Transplantation of Human Amniotic Mesenchymal Stem Cells Promotes Functional Recovery in a Rat Model of Traumatic Spinal Cord Injury. Neurochem Res. 2016; 41(10):2708-2718. [13] WANG M, ZHAI P, CHEN X, et al.Bioengineered scaffolds for spinal cord repair. Tissue Eng Part B Rev. 2011;17(3):177-194. [14] LANG BT, CREGG JM, DEPAUL MA, et al. Modulation of the proteoglycan receptor PTPσ promotes recovery after spinal cord injury. Nature. 2015; 518(7539):404-408. [15] JIANG T, REN XJ, TANG JL, et al. Preparation and characterization of genipin-crosslinked rat acellular spinal cord scaffolds. Mater Sci Eng C Mater Biol Appl. 2013;33(6):3514-3521. [16] WANG YH, CHEN J, ZHOU J, et al. Reduced inflammatory cell recruitment and tissue damage in spinal cord injury by acellular spinal cord scaffold seeded with mesenchymal stem cells. Exp Ther Med. 2017;13(1):203-207. [17] BAN DX, LIU Y, CAO TW, et al. The preparation of rat’s acellular spinal cord scaffold and co-culture with rat’s spinal cord neuron in vitro. Spinal Cord. 2017;55(4):411-418. [18] LIU J, LI K, ZHOU J, et al. Bisperoxovanadium induces M2-type macrophages and promotes functional recovery after spinal cord injury. Mol Immunol. 2019;116:56-62. [19] XING H, REN X, YIN H, et al. Construction of a NT-3 sustained-release system cross-linked with an acellular spinal cord scaffold and its effects on differentiation of cultured bone marrow mesenchymal stem cells. Mater Sci Eng C Mater Biol Appl. 2019;104:109902. [20] XU HL, TIAN FR, LU CT, et al. Thermo-sensitive hydrogels combined with decellularised matrix deliver bFGF for the functional recovery of rats after a spinal cord injury. Sci Rep. 2016;6:38332. [21] XU ZX, ZHANG LQ, WANG CS, et al. Acellular Spinal Cord Scaffold Implantation Promotes Vascular Remodeling with Sustained Delivery of VEGF in a Rat Spinal Cord Hemisection Model. Curr Neurovasc Res. 2017; 14(3):274-289. [22] ZHAO F, GRAYSON WL, MA T, et al. Effects of hydroxyapatite in 3-D chitosan-gelatin polymer network on human mesenchymal stem cell construct development. Biomaterials. 2011;27(9):1859-1867. [23] CHEDLY J, SOARES S, MONTEMBAULT A, et al. Physical chitosan microhydrogels as scaffolds for spinal cord injury restoration and axon regeneration. Biomaterials. 2017;138:91-107. [24] NOMURA H, BALADIE B, KATAYAMA Y, et al. Delayed implantation of intramedullary chitosan channels containing nerve grafts promotes extensive axonal regeneration after spinal cord injury. Neurosurgery. 2008;63(1):141-143. [25] YAO ZA, CHEN FJ, CUI HL, et al. Efficacy of chitosan and sodium alginate scaffolds for repair of spinal cord injury in rats. Neural Regen Res. 2018; 13(3):502-509. [26] 刘东,朱冬昀,彭长亮,等.脊髓损伤修复的复合透明质酸水凝胶支架的构建及其评价[J].山东大学学报(医学版),2017,55(9):53-59. [27] MUKHAMEDSHINA YO, AKHMETZYANOVA ER, KOSTENNIKOV AA, et al. Adipose-Derived Mesenchymal Stem Cell Application Combined With Fibrin Matrix Promotes Structural and Functional Recovery Following Spinal Cord Injury in Rats. Front Pharmacol. 2018;9:343. [28] SHARP KG, DICKSON AR, MARCHENKO SA, et al.Salmon fibrin treatment of spinal cord injury promotes functional recovery and density of serotonergic innervation. Exp Neurol. 2012;235(1):345-356. [29] YAO S, YU S, CAO Z, et al. Hierarchically aligned fibrin nanofiber hydrogel accelerated axonal regrowth and locomotor function recovery in rat spinal cord injury. Int J Nanomedicine. 2018;13:2883-2895. [30] JALALI MONFARED M, NASIRINEZHAD F, EBRAHIMI-BAROUGH S, et al. Transplantation of miR-219 overexpressed human endometrial stem cells encapsulated in fibrin hydrogel in spinal cord injury. J Cell Physiol. 2019;234(10):18887-18896. [31] CHEN X, ZHAO Y, LI X, et al. Functional Multichannel Poly(Propylene Fumarate)-Collagen Scaffold with Collagen-Binding Neurotrophic Factor 3 Promotes Neural Regeneration After Transected Spinal Cord Injury. Adv Healthc Mater. 2018;7(14):e1800315. [32] WANG L, SHI Q, DAI J, et al. Increased vascularization promotes functional recovery in the transected spinal cord rats by implanted vascular endothelial growth factor-targeting collagen scaffold. J Orthop Res. 2018; 36(3):1024-1034. [33] SHI Q, GAO W, HAN X, et al. Collagen scaffolds modified with collagen-binding bFGF promotes the neural regeneration in a rat hemisected spinal cord injury model. Sci China Life Sci. 2014;57(2):232-340. [34] WANG N, XIAO Z, ZHAO Y, et al. Collagen scaffold combined with human umbilical cord-derived mesenchymal stem cells promote functional recovery after scar resection in rats with chronic spinal cord injury. J Tissue Eng Regen Med. 2018;12(2):e1154-e1163. [35] FAN C, LI X, XIAO Z, et al. A modified collagen scaffold facilitates endogenous neurogenesis for acute spinal cord injury repair. Acta Biomater. 2017;51: 304-316. [36] ZHOU X, SHI G, FAN B, et al. Polycaprolactone electrospun fiber scaffold loaded with iPSCs-NSCs and ASCs as a novel tissue engineering scaffold for the treatment of spinal cord injury. Int J Nanomedicine0 2018;13:6265-6277. [37] ZHANG S, WANG XJ, LI WS, et al. Polycaprolactone/polysialic acid hybrid, multifunctional nanofiber scaffolds for treatment of spinal cord injury. Acta Biomater. 2018;77:15-27. [38] BABALOO H, EBRAHIMI-BAROUGH S, DERAKHSHAN MA, et al. PCL/gelatin nanofibrous scaffolds with human endometrial stem cells/Schwann cells facilitate axon regeneration in spinal cord injury. J Cell Physiol. 2019;234(7): 11060-11069. [39] TERRAF P, KOUHSARI SM, AI J, et al. Tissue-Engineered Regeneration of Hemisected Spinal Cord Using Human Endometrial Stem Cells, Poly ¦Å-C aprolactone Scaffolds, and Crocin as a Neuroprotective Agent. Mol Neurobiol. 2017;54(7):5657-5667. [40] SHU B, LIU XB, ZHOU JF, et al. Polypyrrole/polylactic acid nanofibrous scaffold cotransplanted with bone marrow stromal cells promotes the functional recovery of spinal cord injury in rats. CNS Neurosci Ther. 2019; 25(9):951-964. [41] SANTHOSH KT, ALIZADEH A, KARIMI-ABDOLREZAEE S. Design and optimization of PLGA microparticles for controlled and local delivery of Neuregulin-1 in traumatic spinal cord injury. J Control Release. 2017;261: 147-162. [42] PAN S, QI Z, LI Q, et al. Graphene oxide-PLGA hybrid nanofibres for the local delivery of IGF-1 and BDNF in spinal cord repair. Artif Cells Nanomed Biotechnol. 2019;47(1):651-664. [43] WEN Y, YU S, WU Y, et al. Spinal cord injury repair by implantation of structured hyaluronic acid scaffold with PLGA microspheres in the rat. Cell Tissue Res. 2016;364(1):17-28. [44] WANG C, SUN C, HU Z, et al. Improved Neural Regeneration with Olfactory Ensheathing Cell Inoculated PLGA Scaffolds in Spinal Cord Injury Adult Rats. Neurosignals. 2017;25(1):1-14. [45] CALDWELL AS, RAO VV, GOLDEN AC, et al. Porous bio-click microgel scaffolds control hMSC interactions and promote their secretory properties. Biomaterials. 2020;232:119725. [46] SHI R. Polyethylene glycol repairs membrane damage and enhances functional recovery: a tissue engineering ap proach to spinal cord injury. Neurosci Bull. 2013;29(4):460-466. [47] KIM CY. PEG-assisted reconstruction of the cervical spinal cord in rats: effects on motor conduction at 1h. Spinal Cord. 2016;54(10):910-912. [48] 朱旭,于国渊,杨华堂,等.胶原-壳聚糖支架对脊髓损伤后运动功能恢复作用的实验研究[J].中国实用神经疾病杂志,2020,23(23):2032-2038. [49] WANG C, YUE H, FENG Q, et al. Injectable Nanoreinforced Shape-Memory Hydrogel System for Regenerating Spinal Cord Tissue from Traumatic Injury. ACS Appl Mater Interfaces. 2018;10(35):29299-29307. [50] HAN GH, KIM SJ, KO WK, et al. Injectable Hydrogel Containing Tauroursodeoxycholic Acid for Anti-neuroinflammatory Therapy After Spinal Cord Injury in Rats. Mol Neurobiol. 2020;57(10):4007-4017. [51] 赵宣淇,张钰,秦川,等.神经营养因子-3基因修饰的骨髓间充质干细胞和水凝胶联合应用对脊髓损伤模型大鼠的治疗作用研究[J].中国比较医学杂志,2020,30(7):1-12. [52] NAZEMI Z, NOURBAKHSH MS, KIANI S, et al. Co-delivery of minocycline and paclitaxel from injectable hydrogel for treatment of spinal cord injury. J Control Release. 2020;321:145-158. [53] ALBASHARI A, HE Y, ZHANG Y, et al. Thermosensitive BFGF-Modified Hydrogel with Dental Pulp Stem Cells on Neuroinflammation of Spinal Cord Injury. ACS Omega. 2020;5(26):16064-16075. [54] HU X, LI R, WU Y, et al. Thermosensitive heparin-poloxamer hydrogel encapsulated bFGF and NGF to treat spinal cord injury . J Cell Mol Med. 2020;24(14):8166-8178. [55] 王维,齐社宁,赵红斌,等.地塞米松复合聚己内酯胶原支架材料的构建及性能评价[J].中国组织工程研究,2016,20(3):402-407.
[1] AHUJA CS, NORI S, TETREAULT L, et al. Traumatic Spinal Cord Injury-Repair and Regeneration. Neurosurgery. 2017;80(3s):S9-s22.
[2] WU J, LIPINSKI MM. Autophagy in Neurotrauma: Good, Bad, or Dysregulated. Cells. 2019;8(7):693.
[3] FAN B, WEI Z, YAO X, et al. Microenvironment Imbalance of Spinal Cord Injury. Cell Transplant. 2018;27(6):853-866.
[4] COFANO F, BOIDO M, MONTICELLI M, et al. Mesenchymal Stem Cells for Spinal Cord Injury: Current Options, Limitations, and Future of Cell Therapy. Int J Mol Sci. 2019;20(11):2698.
[5] 刘佳.脊髓脱细胞支架复合人脐血间充质干细胞促进大鼠脊髓长节段缺损轴突长入再生及功能恢复[D].广州:南方医科大学,2013.
[6] KATOH H, YOKOTA K, FEHLINGS MG. Regeneration of Spinal Cord Connectivity Through Stem Cell Transplantation and Biomaterial Scaffolds. Front Cell Neurosci. 2019;13:248.
[7] LI X, LIU D, XIAO Z, et al. Scaffold-facilitated locomotor improvement post complete spinal cord injury: Motor axon regeneration versus endogenous neuronal relay formation. Biomaterials. 2019;197:20-31.
[8] DEAL DN, GRIFFIN JW, HOGAN MV. Nerve conduits for nerve repair or reconstruction. J Am Acad Orthop Surg. 2012;20(2):63-68.
[9] CHEN JC, LI LM, GAO JQ. Biomaterials for local drug delivery in central nervous system. Int J Pharm. 2019;560:92-100.
[10] ZHANG J, YUN S, BI J, et al. Enhanced multi-lineage differentiation of human mesenchymal stem/stromal cells within poly(N-isopropy lacrylamide-acrylic acid) microgel-formed three-dimensional constructs. J Mater Chem B Mater Biol Med. 2018;6(12):1799-1814.
[11] OLIVEIRA MB, RIBEIRO MP, MIGUEL SP, et al. In vivo high-content evaluation of three-dimensional scaffolds biocompatibility. Tissue Eng Part C Methods. 2014;20(11):851-864.
[12] ZHOU HL, ZHANG XJ, ZHANG MY, et al. Transplantation of Human Amniotic Mesenchymal Stem Cells Promotes Functional Recovery in a Rat Model of Traumatic Spinal Cord Injury. Neurochem Res. 2016; 41(10):2708-2718.
[13] WANG M, ZHAI P, CHEN X, et al.Bioengineered scaffolds for spinal cord repair. Tissue Eng Part B Rev. 2011;17(3):177-194.
[14] LANG BT, CREGG JM, DEPAUL MA, et al. Modulation of the proteoglycan receptor PTPσ promotes recovery after spinal cord injury. Nature. 2015; 518(7539):404-408.
[15] JIANG T, REN XJ, TANG JL, et al. Preparation and characterization of genipin-crosslinked rat acellular spinal cord scaffolds. Mater Sci Eng C Mater Biol Appl. 2013;33(6):3514-3521.
[16] WANG YH, CHEN J, ZHOU J, et al. Reduced inflammatory cell recruitment and tissue damage in spinal cord injury by acellular spinal cord scaffold seeded with mesenchymal stem cells. Exp Ther Med. 2017;13(1):203-207.
[17] BAN DX, LIU Y, CAO TW, et al. The preparation of rat’s acellular spinal cord scaffold and co-culture with rat’s spinal cord neuron in vitro. Spinal Cord. 2017;55(4):411-418.
[18] LIU J, LI K, ZHOU J, et al. Bisperoxovanadium induces M2-type macrophages and promotes functional recovery after spinal cord injury. Mol Immunol. 2019;116:56-62.
[19] XING H, REN X, YIN H, et al. Construction of a NT-3 sustained-release system cross-linked with an acellular spinal cord scaffold and its effects on differentiation of cultured bone marrow mesenchymal stem cells. Mater Sci Eng C Mater Biol Appl. 2019;104:109902.
[20] XU HL, TIAN FR, LU CT, et al. Thermo-sensitive hydrogels combined with decellularised matrix deliver bFGF for the functional recovery of rats after a spinal cord injury. Sci Rep. 2016;6:38332.
[21] XU ZX, ZHANG LQ, WANG CS, et al. Acellular Spinal Cord Scaffold Implantation Promotes Vascular Remodeling with Sustained Delivery of VEGF in a Rat Spinal Cord Hemisection Model. Curr Neurovasc Res. 2017; 14(3):274-289.
[22] ZHAO F, GRAYSON WL, MA T, et al. Effects of hydroxyapatite in 3-D chitosan-gelatin polymer network on human mesenchymal stem cell construct development. Biomaterials. 2011;27(9):1859-1867.
[23] CHEDLY J, SOARES S, MONTEMBAULT A, et al. Physical chitosan microhydrogels as scaffolds for spinal cord injury restoration and axon regeneration. Biomaterials. 2017;138:91-107.
[24] NOMURA H, BALADIE B, KATAYAMA Y, et al. Delayed implantation of intramedullary chitosan channels containing nerve grafts promotes extensive axonal regeneration after spinal cord injury. Neurosurgery. 2008;63(1):141-143.
[25] YAO ZA, CHEN FJ, CUI HL, et al. Efficacy of chitosan and sodium alginate scaffolds for repair of spinal cord injury in rats. Neural Regen Res. 2018; 13(3):502-509.
[26] 刘东,朱冬昀,彭长亮,等.脊髓损伤修复的复合透明质酸水凝胶支架的构建及其评价[J].山东大学学报(医学版),2017,55(9):53-59.
[27] MUKHAMEDSHINA YO, AKHMETZYANOVA ER, KOSTENNIKOV AA, et al. Adipose-Derived Mesenchymal Stem Cell Application Combined With Fibrin Matrix Promotes Structural and Functional Recovery Following Spinal Cord Injury in Rats. Front Pharmacol. 2018;9:343.
[28] SHARP KG, DICKSON AR, MARCHENKO SA, et al.Salmon fibrin treatment of spinal cord injury promotes functional recovery and density of serotonergic innervation. Exp Neurol. 2012;235(1):345-356.
[29] YAO S, YU S, CAO Z, et al. Hierarchically aligned fibrin nanofiber hydrogel accelerated axonal regrowth and locomotor function recovery in rat spinal cord injury. Int J Nanomedicine. 2018;13:2883-2895.
[30] JALALI MONFARED M, NASIRINEZHAD F, EBRAHIMI-BAROUGH S, et al. Transplantation of miR-219 overexpressed human endometrial stem cells encapsulated in fibrin hydrogel in spinal cord injury. J Cell Physiol. 2019;234(10):18887-18896.
[31] CHEN X, ZHAO Y, LI X, et al. Functional Multichannel Poly(Propylene Fumarate)-Collagen Scaffold with Collagen-Binding Neurotrophic Factor 3 Promotes Neural Regeneration After Transected Spinal Cord Injury. Adv Healthc Mater. 2018;7(14):e1800315.
[32] WANG L, SHI Q, DAI J, et al. Increased vascularization promotes functional recovery in the transected spinal cord rats by implanted vascular endothelial growth factor-targeting collagen scaffold. J Orthop Res. 2018; 36(3):1024-1034.
[33] SHI Q, GAO W, HAN X, et al. Collagen scaffolds modified with collagen-binding bFGF promotes the neural regeneration in a rat hemisected spinal cord injury model. Sci China Life Sci. 2014;57(2):232-340.
[34] WANG N, XIAO Z, ZHAO Y, et al. Collagen scaffold combined with human umbilical cord-derived mesenchymal stem cells promote functional recovery after scar resection in rats with chronic spinal cord injury. J Tissue Eng Regen Med. 2018;12(2):e1154-e1163.
[35] FAN C, LI X, XIAO Z, et al. A modified collagen scaffold facilitates endogenous neurogenesis for acute spinal cord injury repair. Acta Biomater. 2017;51: 304-316.
[36] ZHOU X, SHI G, FAN B, et al. Polycaprolactone electrospun fiber scaffold loaded with iPSCs-NSCs and ASCs as a novel tissue engineering scaffold for the treatment of spinal cord injury. Int J Nanomedicine0 2018;13:6265-6277.
[37] ZHANG S, WANG XJ, LI WS, et al. Polycaprolactone/polysialic acid hybrid, multifunctional nanofiber scaffolds for treatment of spinal cord injury. Acta Biomater. 2018;77:15-27.
[38] BABALOO H, EBRAHIMI-BAROUGH S, DERAKHSHAN MA, et al. PCL/gelatin nanofibrous scaffolds with human endometrial stem cells/Schwann cells facilitate axon regeneration in spinal cord injury. J Cell Physiol. 2019;234(7): 11060-11069.
[39] TERRAF P, KOUHSARI SM, AI J, et al. Tissue-Engineered Regeneration of Hemisected Spinal Cord Using Human Endometrial Stem Cells, Poly ¦Å-C aprolactone Scaffolds, and Crocin as a Neuroprotective Agent. Mol Neurobiol. 2017;54(7):5657-5667.
[40] SHU B, LIU XB, ZHOU JF, et al. Polypyrrole/polylactic acid nanofibrous scaffold cotransplanted with bone marrow stromal cells promotes the functional recovery of spinal cord injury in rats. CNS Neurosci Ther. 2019; 25(9):951-964.
[41] SANTHOSH KT, ALIZADEH A, KARIMI-ABDOLREZAEE S. Design and optimization of PLGA microparticles for controlled and local delivery of Neuregulin-1 in traumatic spinal cord injury. J Control Release. 2017;261: 147-162.
[42] PAN S, QI Z, LI Q, et al. Graphene oxide-PLGA hybrid nanofibres for the local delivery of IGF-1 and BDNF in spinal cord repair. Artif Cells Nanomed Biotechnol. 2019;47(1):651-664.
[43] WEN Y, YU S, WU Y, et al. Spinal cord injury repair by implantation of structured hyaluronic acid scaffold with PLGA microspheres in the rat. Cell Tissue Res. 2016;364(1):17-28.
[44] WANG C, SUN C, HU Z, et al. Improved Neural Regeneration with Olfactory Ensheathing Cell Inoculated PLGA Scaffolds in Spinal Cord Injury Adult Rats. Neurosignals. 2017;25(1):1-14.
[45] CALDWELL AS, RAO VV, GOLDEN AC, et al. Porous bio-click microgel scaffolds control hMSC interactions and promote their secretory properties. Biomaterials. 2020;232:119725.
[46] SHI R. Polyethylene glycol repairs membrane damage and enhances functional recovery: a tissue engineering ap proach to spinal cord injury. Neurosci Bull. 2013;29(4):460-466.
[47] KIM CY. PEG-assisted reconstruction of the cervical spinal cord in rats: effects on motor conduction at 1h. Spinal Cord. 2016;54(10):910-912.
[48] 朱旭,于国渊,杨华堂,等.胶原-壳聚糖支架对脊髓损伤后运动功能恢复作用的实验研究[J].中国实用神经疾病杂志,2020,23(23):2032-2038.
[49] WANG C, YUE H, FENG Q, et al. Injectable Nanoreinforced Shape-Memory Hydrogel System for Regenerating Spinal Cord Tissue from Traumatic Injury. ACS Appl Mater Interfaces. 2018;10(35):29299-29307.
[50] HAN GH, KIM SJ, KO WK, et al. Injectable Hydrogel Containing Tauroursodeoxycholic Acid for Anti-neuroinflammatory Therapy After Spinal Cord Injury in Rats. Mol Neurobiol. 2020;57(10):4007-4017.
[51] 赵宣淇,张钰,秦川,等.神经营养因子-3基因修饰的骨髓间充质干细胞和水凝胶联合应用对脊髓损伤模型大鼠的治疗作用研究[J].中国比较医学杂志,2020,30(7):1-12.
[52] NAZEMI Z, NOURBAKHSH MS, KIANI S, et al. Co-delivery of minocycline and paclitaxel from injectable hydrogel for treatment of spinal cord injury. J Control Release. 2020;321:145-158.
[53] ALBASHARI A, HE Y, ZHANG Y, et al. Thermosensitive BFGF-Modified Hydrogel with Dental Pulp Stem Cells on Neuroinflammation of Spinal Cord Injury. ACS Omega. 2020;5(26):16064-16075.
[54] HU X, LI R, WU Y, et al. Thermosensitive heparin-poloxamer hydrogel encapsulated bFGF and NGF to treat spinal cord injury . J Cell Mol Med. 2020;24(14):8166-8178.
[55] 王维,齐社宁,赵红斌,等.地塞米松复合聚己内酯胶原支架材料的构建及性能评价[J].中国组织工程研究,2016,20(3):402-407.
[1] AHUJA CS, NORI S, TETREAULT L, et al. Traumatic Spinal Cord Injury-Repair and Regeneration. Neurosurgery. 2017;80(3s):S9-s22.
[2] WU J, LIPINSKI MM. Autophagy in Neurotrauma: Good, Bad, or Dysregulated. Cells. 2019;8(7):693.
[3] FAN B, WEI Z, YAO X, et al. Microenvironment Imbalance of Spinal Cord Injury. Cell Transplant. 2018;27(6):853-866.
[4] COFANO F, BOIDO M, MONTICELLI M, et al. Mesenchymal Stem Cells for Spinal Cord Injury: Current Options, Limitations, and Future of Cell Therapy. Int J Mol Sci. 2019;20(11):2698.
[5] 刘佳.脊髓脱细胞支架复合人脐血间充质干细胞促进大鼠脊髓长节段缺损轴突长入再生及功能恢复[D].广州:南方医科大学,2013.
[6] KATOH H, YOKOTA K, FEHLINGS MG. Regeneration of Spinal Cord Connectivity Through Stem Cell Transplantation and Biomaterial Scaffolds. Front Cell Neurosci. 2019;13:248.
[7] LI X, LIU D, XIAO Z, et al. Scaffold-facilitated locomotor improvement post complete spinal cord injury: Motor axon regeneration versus endogenous neuronal relay formation. Biomaterials. 2019;197:20-31.
[8] DEAL DN, GRIFFIN JW, HOGAN MV. Nerve conduits for nerve repair or reconstruction. J Am Acad Orthop Surg. 2012;20(2):63-68.
[9] CHEN JC, LI LM, GAO JQ. Biomaterials for local drug delivery in central nervous system. Int J Pharm. 2019;560:92-100.
[10] ZHANG J, YUN S, BI J, et al. Enhanced multi-lineage differentiation of human mesenchymal stem/stromal cells within poly(N-isopropy lacrylamide-acrylic acid) microgel-formed three-dimensional constructs. J Mater Chem B Mater Biol Med. 2018;6(12):1799-1814.
[11] OLIVEIRA MB, RIBEIRO MP, MIGUEL SP, et al. In vivo high-content evaluation of three-dimensional scaffolds biocompatibility. Tissue Eng Part C Methods. 2014;20(11):851-864.
[12] ZHOU HL, ZHANG XJ, ZHANG MY, et al. Transplantation of Human Amniotic Mesenchymal Stem Cells Promotes Functional Recovery in a Rat Model of Traumatic Spinal Cord Injury. Neurochem Res. 2016; 41(10):2708-2718.
[13] WANG M, ZHAI P, CHEN X, et al.Bioengineered scaffolds for spinal cord repair. Tissue Eng Part B Rev. 2011;17(3):177-194.
[14] LANG BT, CREGG JM, DEPAUL MA, et al. Modulation of the proteoglycan receptor PTPσ promotes recovery after spinal cord injury. Nature. 2015; 518(7539):404-408.
[15] JIANG T, REN XJ, TANG JL, et al. Preparation and characterization of genipin-crosslinked rat acellular spinal cord scaffolds. Mater Sci Eng C Mater Biol Appl. 2013;33(6):3514-3521.
[16] WANG YH, CHEN J, ZHOU J, et al. Reduced inflammatory cell recruitment and tissue damage in spinal cord injury by acellular spinal cord scaffold seeded with mesenchymal stem cells. Exp Ther Med. 2017;13(1):203-207.
[17] BAN DX, LIU Y, CAO TW, et al. The preparation of rat’s acellular spinal cord scaffold and co-culture with rat’s spinal cord neuron in vitro. Spinal Cord. 2017;55(4):411-418.
[18] LIU J, LI K, ZHOU J, et al. Bisperoxovanadium induces M2-type macrophages and promotes functional recovery after spinal cord injury. Mol Immunol. 2019;116:56-62.
[19] XING H, REN X, YIN H, et al. Construction of a NT-3 sustained-release system cross-linked with an acellular spinal cord scaffold and its effects on differentiation of cultured bone marrow mesenchymal stem cells. Mater Sci Eng C Mater Biol Appl. 2019;104:109902.
[20] XU HL, TIAN FR, LU CT, et al. Thermo-sensitive hydrogels combined with decellularised matrix deliver bFGF for the functional recovery of rats after a spinal cord injury. Sci Rep. 2016;6:38332.
[21] XU ZX, ZHANG LQ, WANG CS, et al. Acellular Spinal Cord Scaffold Implantation Promotes Vascular Remodeling with Sustained Delivery of VEGF in a Rat Spinal Cord Hemisection Model. Curr Neurovasc Res. 2017; 14(3):274-289.
[22] ZHAO F, GRAYSON WL, MA T, et al. Effects of hydroxyapatite in 3-D chitosan-gelatin polymer network on human mesenchymal stem cell construct development. Biomaterials. 2011;27(9):1859-1867.
[23] CHEDLY J, SOARES S, MONTEMBAULT A, et al. Physical chitosan microhydrogels as scaffolds for spinal cord injury restoration and axon regeneration. Biomaterials. 2017;138:91-107.
[24] NOMURA H, BALADIE B, KATAYAMA Y, et al. Delayed implantation of intramedullary chitosan channels containing nerve grafts promotes extensive axonal regeneration after spinal cord injury. Neurosurgery. 2008;63(1):141-143.
[25] YAO ZA, CHEN FJ, CUI HL, et al. Efficacy of chitosan and sodium alginate scaffolds for repair of spinal cord injury in rats. Neural Regen Res. 2018; 13(3):502-509.
[26] 刘东,朱冬昀,彭长亮,等.脊髓损伤修复的复合透明质酸水凝胶支架的构建及其评价[J].山东大学学报(医学版),2017,55(9):53-59.
[27] MUKHAMEDSHINA YO, AKHMETZYANOVA ER, KOSTENNIKOV AA, et al. Adipose-Derived Mesenchymal Stem Cell Application Combined With Fibrin Matrix Promotes Structural and Functional Recovery Following Spinal Cord Injury in Rats. Front Pharmacol. 2018;9:343.
[28] SHARP KG, DICKSON AR, MARCHENKO SA, et al.Salmon fibrin treatment of spinal cord injury promotes functional recovery and density of serotonergic innervation. Exp Neurol. 2012;235(1):345-356.
[29] YAO S, YU S, CAO Z, et al. Hierarchically aligned fibrin nanofiber hydrogel accelerated axonal regrowth and locomotor function recovery in rat spinal cord injury. Int J Nanomedicine. 2018;13:2883-2895.
[30] JALALI MONFARED M, NASIRINEZHAD F, EBRAHIMI-BAROUGH S, et al. Transplantation of miR-219 overexpressed human endometrial stem cells encapsulated in fibrin hydrogel in spinal cord injury. J Cell Physiol. 2019;234(10):18887-18896.
[31] CHEN X, ZHAO Y, LI X, et al. Functional Multichannel Poly(Propylene Fumarate)-Collagen Scaffold with Collagen-Binding Neurotrophic Factor 3 Promotes Neural Regeneration After Transected Spinal Cord Injury. Adv Healthc Mater. 2018;7(14):e1800315.
[32] WANG L, SHI Q, DAI J, et al. Increased vascularization promotes functional recovery in the transected spinal cord rats by implanted vascular endothelial growth factor-targeting collagen scaffold. J Orthop Res. 2018; 36(3):1024-1034.
[33] SHI Q, GAO W, HAN X, et al. Collagen scaffolds modified with collagen-binding bFGF promotes the neural regeneration in a rat hemisected spinal cord injury model. Sci China Life Sci. 2014;57(2):232-340.
[34] WANG N, XIAO Z, ZHAO Y, et al. Collagen scaffold combined with human umbilical cord-derived mesenchymal stem cells promote functional recovery after scar resection in rats with chronic spinal cord injury. J Tissue Eng Regen Med. 2018;12(2):e1154-e1163.
[35] FAN C, LI X, XIAO Z, et al. A modified collagen scaffold facilitates endogenous neurogenesis for acute spinal cord injury repair. Acta Biomater. 2017;51: 304-316.
[36] ZHOU X, SHI G, FAN B, et al. Polycaprolactone electrospun fiber scaffold loaded with iPSCs-NSCs and ASCs as a novel tissue engineering scaffold for the treatment of spinal cord injury. Int J Nanomedicine0 2018;13:6265-6277.
[37] ZHANG S, WANG XJ, LI WS, et al. Polycaprolactone/polysialic acid hybrid, multifunctional nanofiber scaffolds for treatment of spinal cord injury. Acta Biomater. 2018;77:15-27.
[38] BABALOO H, EBRAHIMI-BAROUGH S, DERAKHSHAN MA, et al. PCL/gelatin nanofibrous scaffolds with human endometrial stem cells/Schwann cells facilitate axon regeneration in spinal cord injury. J Cell Physiol. 2019;234(7): 11060-11069.
[39] TERRAF P, KOUHSARI SM, AI J, et al. Tissue-Engineered Regeneration of Hemisected Spinal Cord Using Human Endometrial Stem Cells, Poly ¦Å-C aprolactone Scaffolds, and Crocin as a Neuroprotective Agent. Mol Neurobiol. 2017;54(7):5657-5667.
[40] SHU B, LIU XB, ZHOU JF, et al. Polypyrrole/polylactic acid nanofibrous scaffold cotransplanted with bone marrow stromal cells promotes the functional recovery of spinal cord injury in rats. CNS Neurosci Ther. 2019; 25(9):951-964.
[41] SANTHOSH KT, ALIZADEH A, KARIMI-ABDOLREZAEE S. Design and optimization of PLGA microparticles for controlled and local delivery of Neuregulin-1 in traumatic spinal cord injury. J Control Release. 2017;261: 147-162.
[42] PAN S, QI Z, LI Q, et al. Graphene oxide-PLGA hybrid nanofibres for the local delivery of IGF-1 and BDNF in spinal cord repair. Artif Cells Nanomed Biotechnol. 2019;47(1):651-664.
[43] WEN Y, YU S, WU Y, et al. Spinal cord injury repair by implantation of structured hyaluronic acid scaffold with PLGA microspheres in the rat. Cell Tissue Res. 2016;364(1):17-28.
[44] WANG C, SUN C, HU Z, et al. Improved Neural Regeneration with Olfactory Ensheathing Cell Inoculated PLGA Scaffolds in Spinal Cord Injury Adult Rats. Neurosignals. 2017;25(1):1-14.
[45] CALDWELL AS, RAO VV, GOLDEN AC, et al. Porous bio-click microgel scaffolds control hMSC interactions and promote their secretory properties. Biomaterials. 2020;232:119725.
[46] SHI R. Polyethylene glycol repairs membrane damage and enhances functional recovery: a tissue engineering ap proach to spinal cord injury. Neurosci Bull. 2013;29(4):460-466.
[47] KIM CY. PEG-assisted reconstruction of the cervical spinal cord in rats: effects on motor conduction at 1h. Spinal Cord. 2016;54(10):910-912.
[48] 朱旭,于国渊,杨华堂,等.胶原-壳聚糖支架对脊髓损伤后运动功能恢复作用的实验研究[J].中国实用神经疾病杂志,2020,23(23):2032-2038.
[49] WANG C, YUE H, FENG Q, et al. Injectable Nanoreinforced Shape-Memory Hydrogel System for Regenerating Spinal Cord Tissue from Traumatic Injury. ACS Appl Mater Interfaces. 2018;10(35):29299-29307.
[50] HAN GH, KIM SJ, KO WK, et al. Injectable Hydrogel Containing Tauroursodeoxycholic Acid for Anti-neuroinflammatory Therapy After Spinal Cord Injury in Rats. Mol Neurobiol. 2020;57(10):4007-4017.
[51] 赵宣淇,张钰,秦川,等.神经营养因子-3基因修饰的骨髓间充质干细胞和水凝胶联合应用对脊髓损伤模型大鼠的治疗作用研究[J].中国比较医学杂志,2020,30(7):1-12.
[52] NAZEMI Z, NOURBAKHSH MS, KIANI S, et al. Co-delivery of minocycline and paclitaxel from injectable hydrogel for treatment of spinal cord injury. J Control Release. 2020;321:145-158.
[53] ALBASHARI A, HE Y, ZHANG Y, et al. Thermosensitive BFGF-Modified Hydrogel with Dental Pulp Stem Cells on Neuroinflammation of Spinal Cord Injury. ACS Omega. 2020;5(26):16064-16075.
[54] HU X, LI R, WU Y, et al. Thermosensitive heparin-poloxamer hydrogel encapsulated bFGF and NGF to treat spinal cord injury . J Cell Mol Med. 2020;24(14):8166-8178.
[55] 王维,齐社宁,赵红斌,等.地塞米松复合聚己内酯胶原支架材料的构建及性能评价[J].中国组织工程研究,2016,20(3):402-407.
|
| [1] | Zhu Chan, Han Xuke, Yao Chengjiao, Zhou Qian, Zhang Qiang, Chen Qiu. Human salivary components and osteoporosis/osteopenia [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1439-1444. |
| [2] | Jin Tao, Liu Lin, Zhu Xiaoyan, Shi Yucong, Niu Jianxiong, Zhang Tongtong, Wu Shujin, Yang Qingshan. Osteoarthritis and mitochondrial abnormalities [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1452-1458. |
| [3] | Zhang Lichuang, Xu Hao, Ma Yinghui, Xiong Mengting, Han Haihui, Bao Jiamin, Zhai Weitao, Liang Qianqian. Mechanism and prospects of regulating lymphatic reflux function in the treatment of rheumatoid arthritis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1459-1466. |
| [4] | Wang Jing, Xiong Shan, Cao Jin, Feng Linwei, Wang Xin. Role and mechanism of interleukin-3 in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1260-1265. |
| [5] | Zhu Chan, Han Xuke, Yao Chengjiao, Zhang Qiang, Liu Jing, Shao Ming. Acupuncture for Parkinson’s disease: an insight into the action mechanism in animal experiments [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1272-1277. |
| [6] | Kan Houming, Fan Lijun, Chen Xuetai, Shen Wen. Application of platelet-rich plasma in neuropathic pain [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1286-1292. |
| [7] | Hui Xiaoshan, Bai Jing, Zhou Siyuan, Wang Jie, Zhang Jinsheng, He Qingyong, Meng Peipei. Theoretical mechanism of traditional Chinese medicine theory on stem cell induced differentiation [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1125-1129. |
| [8] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [9] | Fan Yiming, Liu Fangyu, Zhang Hongyu, Li Shuai, Wang Yansong. Serial questions about endogenous neural stem cell response in the ependymal zone after spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1137-1142. |
| [10] | Guo Jia, Ding Qionghua, Liu Ze, Lü Siyi, Zhou Quancheng, Gao Yuhua, Bai Chunyu. Biological characteristics and immunoregulation of exosomes derived from mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1093-1101. |
| [11] | Wu Weiyue, Guo Xiaodong, Bao Chongyun. Application of engineered exosomes in bone repair and regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1102-1106. |
| [12] | Zhou Hongqin, Wu Dandan, Yang Kun, Liu Qi. Exosomes that deliver specific miRNAs can regulate osteogenesis and promote angiogenesis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1107-1112. |
| [13] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [14] | Huang Chenwei, Fei Yankang, Zhu Mengmei, Li Penghao, Yu Bing. Important role of glutathione in stemness and regulation of stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1119-1124. |
| [15] | Hu Wei, Xie Xingqi, Tu Guanjun. Exosomes derived from bone marrow mesenchymal stem cells improve the integrity of the blood-spinal cord barrier after spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 992-998. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||