Chinese Journal of Tissue Engineering Research ›› 2022, Vol. 26 ›› Issue (28): 4569-4574.doi: 10.12307/2022.315
Previous Articles Next Articles
Research progress of gene therapy for vascularization of tissue engineering
Li Guangzhao, Chen Rui, Jia Honglin, Ren Liling
- School of Stomatology, Lanzhou University, Lanzhou 730000, Gansu Province, China
-
Received:2021-02-19Accepted:2021-04-15Online:2022-10-08Published:2022-03-23 -
Contact:Ren Liling, Professor, Master’s supervisor, School of Stomatology, Lanzhou University, Lanzhou 730000, Gansu Province, China -
About author:Li Guangzhao, School of Stomatology, Lanzhou University, Lanzhou 730000, Gansu Province, China -
Supported by:National Natural Science Foundation of China, No. 81670969 (to RLL), No. 81300860 (to RLL); Natural Science Foundation of Gansu Province, China, No. 17JR5RA202 (to RLL)
CLC Number:
Cite this article
Li Guangzhao, Chen Rui, Jia Honglin, Ren Liling. Research progress of gene therapy for vascularization of tissue engineering[J]. Chinese Journal of Tissue Engineering Research, 2022, 26(28): 4569-4574.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
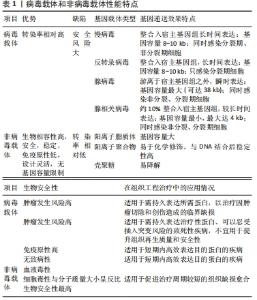
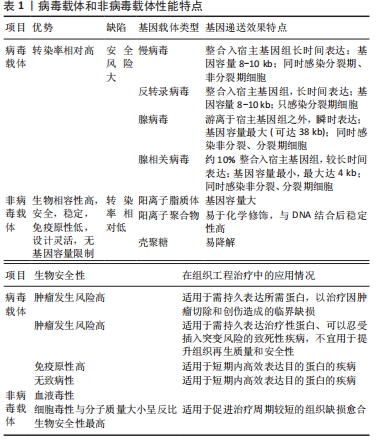
2.1 基因治疗中的成血管种子细胞 基因治疗中促血管化的最基础的种子细胞主要包括:间充质干细胞、内皮细胞和内皮祖细胞。间充质干细胞主要以两种方式实现促组织再生和血管化:在各种因素如基质蛋白、可溶性因子、循环应变、支架等诱导下可塑性地分化为特定类型的功能细胞如成骨细胞、血管内皮细胞和平滑肌细胞,直接参与组织修复[21-23];通过旁分泌机制激活外周受体细胞,参与细胞通讯、细胞迁移、毛细血管发芽,间接参与组织血液循环重建过程[24-25]。因此将各类生长因子基因如骨形态发生蛋白2、转化生长因子β、碱性成纤维细胞生长因子、血管内皮生长因子、血管生成素1、肝细胞生长因子、缺氧诱导因子1α等导入间充质干细胞以构建体内工程化移植物,可以显著放大上述能力[26],在增强间充质干细胞活性的同时提高了其治疗能力,加速了血管化进程[27-31]。 内皮细胞虽然直接参与构成血管,但单一的内皮细胞在基本培养条件下会较快退化,只有在各种血管生成促进剂如碱性成纤维细胞生长因子、血管内皮生长因子和大量胎牛血清等存在的条件下才能观察到血管生成,这恰好为基因治疗的引入提供了用武之地,加之内皮细胞的自分泌作用方式和直接释放基因产物于血管腔内的特点,内皮细胞成为血管化基因治疗中十分有吸引力的种子细胞[32]。研究人员发现当血管内皮生长因子165基因导入内皮细胞后,内皮细胞不仅保持了其固有特性,还表现出良好的增殖和迁移潜能,明显增加了鼠缺血皮瓣中的毛细血管密度[33]。 内皮祖细胞不仅具有分化成毛细血管壁的潜力,也能表达诱导血管生成和组织重塑的分泌因子来促进新血管生成和组织修复[34],与基因修饰技术结合后更是展现出巨大的应用潜力。王娟[35]将转染了血管内皮生长因子164基因的内皮祖细胞移植入脑缺血的大鼠侧脑室中,发现基因修饰后的内皮祖细胞通过HIF-VEGF和SDF-1/CXCR4信号路径促进了鼠脑缺血区的血管新生。薛建新等[36]发现将过表达血管内皮生长因子165基因的内皮祖细胞移植入肾缺血再灌注损伤模型后,该内皮祖细胞不仅在归巢后旁分泌过量血管内皮生长因子,而且进一步促进了血管生成素1、血管生成素2等血管新生因子的表达,从而促进了肾血管新生。汪砚雨[37]也通过研究发现,经过人血管内皮生长因子165基因转染的大鼠内皮祖细胞对血管再生有明显促进作用,并减少了心肌细胞坏死率。 2.2 基因治疗中的促血管化目的基因 血管内皮生长因子、血管生成素1、碱性成纤维细胞生长因子、骨形态发生蛋白2、缺氧诱导因子1α由于各自显著的作用效果,从众多促血管化因子中脱颖而出,在组织工程血管化中备受青睐。通过基因转染实现移植物中血管内皮生长因子的长期表达,可以充分发挥血管内皮生长因子在体内外特异性促进血管内皮细胞增殖与迁移、改善血管通透性及维持血管稳定性等促血管化功能[38]。然而,通过对比血管内皮生长因子基因转染与富血小板血浆治疗兔临界尺寸骨缺损的疗效发现,血管内皮生长因子转染的骨髓间充质干细胞与支架复合移植物能够改善血运重建,而富血小板血浆则可以同时显著增强骨愈合和血管化效果[39],这意味着与血管内皮生长因子单基因治疗相比,血管内皮生长因子结合多种生长因子的基因疗法更有可能提高组织修复的综合效果。 作为血管壁的重要组成成分,周细胞可以通过分泌血管生成素1进一步激活下游信号,从而重塑和稳定血管、增强血管的抗渗透性、提高内皮细胞存活率。Ang-1-Tie-2信号通路主要在血管新生后期促进血管的成熟与稳定,而血管内皮生长因子则负责促进早期管粗、壁薄、缺乏周细胞的母代血管的形成[40],因此血管内皮生长因子和血管生成素1双基因联合转染的移植物能充分发挥二者不同时段的不同成血管效应,比单基因转染组修复骨缺损具有更显著的毛细血管密度和更良好的骨修复效果,同时也防止了移植物微血管渗漏的发生[41]。 碱性成纤维细胞生长因子已被证明主要起两种作用:参与诱导血管生成的全程和促进间充质干细胞及成骨细胞的有丝分裂。碱性成纤维细胞生长因子在促进血管生成方面的效应具体体现在:引发血管生成;促进内皮细胞存活、增殖和迁移;促进血管内皮生长因子受体2稳定表达,从而增加血管稳定性;刺激分泌纤溶酶原激活和胶原酶来降解细胞外基质,使新生毛细血管得以向损伤区域生长[39]。ZHANG等[30]在体外将碱性成纤维细胞生长因子基因转入间充质干细胞,并与支架材料进行复合,然后将其移植入同种异体兔股骨头缺血性坏死区,术后6周发现基因修饰组骨修复面积超过80%,术后12周即完成了全部修复,且新生血管面积密度和新骨形成面积远高于未修饰组。 选择合适的生长因子以实现成骨与成血管的“双赢”,是骨组织工程中的不懈追求。骨形态发生蛋白2是迄今为止被认为最有效的骨诱导因子之一,但近年来研究发现,当骨形态发生蛋白2 在诱导成骨前体细胞向成熟成骨细胞分化时,随着骨形态发生蛋白2浓度的提高和时间的延长,成骨样细胞中血管内皮生长因子的表达也随之增加,进而促进了血管化反应;而血管内皮生长因子不仅能够通过促进血管网络的创建来增加间充质干细胞的募集和存活,而且还能增强间充质干细胞对骨形态发生蛋白2引发的成骨反应能力,二者形成了相互促进、级联放大的关系[42]。但仅依赖骨形态发生蛋白2作用于血管内皮生长因子的间接血管化效率相对较低,因而只靠骨形态发生蛋白2基因诱导血管化不能达到理想的效果,于是研究人员进行了骨形态发生蛋白2/血管内皮生长因子基因联合转染,发现二者的协作关系增强了YAP/TAZ信号传导机制,从而加速了血管化和成骨过程,与单独转染组相比,基因共转染的工程化移植物修复在鼠颅骨和长骨节段性缺损时获得了完全骨愈合[43]。 在促血管化因子被广泛应用的基础上,人们试图利用一种血管发生和进展过程中的上游基因来达到更高效的成血管效果。缺氧诱导因子1α是血管调控网络中的核心转录因子,具有调节血管生成的多个下游因子的能力,由缺氧诱导因子1α基因转染诱导产生的微血管系统具有稳定、无渗漏、无水肿等优点,从而避免了因单独利用下游基因如血管内皮生长因子而产生血管瘤、血管畸形等现象[44]。YING等[45]将转染了突变体缺氧诱导因子1α的大鼠骨髓间充质干细胞接种于β-磷酸三钙支架并植入鼠颅骨临界骨缺损区域,发现基因转染组不仅有效刺激了骨髓间充质干细胞的增殖和成骨分化,而且新血管形成标记物CD31被显著上调,与未转染组相比,缺氧诱导因子1α基因转染的工程化移植物拥有更多、更快的新骨和新生血管形成。除了利用上游基因之外,双/多基因联合转染也可以通过细胞因子之间的协调作用来优化工程化移植物的血管化效果,例如骨形态发生蛋白2基因与碱性成纤维细胞生长因子基因联合转染比单基因修饰组拥有更丰富的毛细血管网[46];血小板衍生生长因子和血管内皮生长因子或成纤维细胞生长因子的双重基因递送可迅速形成成熟的血管网络[47-48],这些实验均表明双/多基因联合递送可能在组织工程血管化中拥有更广阔的前景。 2.3 基因治疗中的载体 选用合适的载体将目的基因高效导入种子细胞是实现工程化组织基因增强的必要前提。目前基因传递系统分为病毒载体和非病毒载体两大类。 强基因转移能力是病毒的固有特性,因而当毒性基因序列被替换为目的基因后即成为高效的基因转移载体,其在基因治疗中占据了约70%的应用空间。不同载体的适用范围因目标细胞类型和期望表达的时间而不同[17]。慢病毒具有可以感染原代细胞、转染效率高、容纳基因量大等优点,但来源和基因组的复杂性限制了其临床应用,因此主要用于需要长期表达目的蛋白以促进临界缺损愈合的肿瘤切除或创伤性疾病。反转录病毒可以随机重组到分裂期细胞的宿主染色体内,持久稳定地表达所需蛋白质,但是这可能会破坏细胞周期进程,通过插入突变导致肿瘤发生,所以不宜用于治疗周期较短的非致死性疾病。腺病毒的宿主细胞范围广,其独特之处是能使目的基因瞬时高效表达并迅速消退,从而减少了外源基因对宿主后期的不良反应,尤其是不整合入宿主基因组,避免了癌变可能,针对其高免疫原性,第3代腺病毒极大降低了免疫原性,因此适用于各种损伤等需要短期内高效表达外源基因的治疗。腺相关病毒具有非常高的安全性,它不引起任何已知的疾病,其不足之处是:由于容量小,且依赖于腺病毒或疱疹病毒存在才能生产出成熟的病毒颗粒,其生产成本和技术复杂性显著增加。尽管病毒载体拥有较高的转染效率,但这是以潜在的致癌风险和针对病毒成分的免疫反应作为代价的,同时其基因装载容量和大规模生产也受到限制[49]。针对以上不足,目前对病毒载体的主要改进目标是提高其转染能力和长期应用的生物安全性[50],例如通过修饰病毒衣壳蛋白以形成靶向性转染及构建无病毒基因的无肠型腺病毒载体等,甚至可以通过组合两种及以上的不同病毒,来扬长避短、构建集多种特性于一体的嵌合病毒载体。 尽管目前大多数实验主要以病毒为媒介进行组织工程血管化基因治疗,但非病毒转移基因法因其具有更高的安全性、易于组装和大规模生产、导入方式多样等优点,正在逐渐成为未来研究的重点[51]。非病毒载体通常被制备成纳米粒子来负载目的基因,主要包括阳离子脂质体、阳离子聚合物如聚乙烯亚胺、生物可降解聚磷脂和壳聚糖等[52]。阳离子脂质体因其独特的脂双层结构而具有生物相容性强、包装容量大的特点,可以成功感染多种细胞;阳离子聚合物与DNA结合后形成多链式的复合体,比脂质复合体更加稳定,其上的多种官能团也易于被化学修饰而获得所需的理化性质[53]。EFS-WITTE等[44]发现,用聚(β-氨基酯)递送缺氧诱导因子1α至人脂肪干细胞可以诱导3D血管形成。壳聚糖是一种生物相容性无可比拟的基因传输系统,RAFTERY等[54]用胶原羟基磷灰石支架负载了携带骨形态发生蛋白2和血管内皮生长因子基因的壳聚糖纳米颗粒,同时将间充质干细胞播种在该支架上,发现间充质干细胞的目的蛋白表达可持续长达28 d,将该支架移植到动物颅骨临界骨缺损区后有效促进了骨与血管新生。非病毒载体最大的缺陷是基因转染效率较低,因此研究者已从多重角度出发来改进其转染效果,如缩小载体尺寸或对纳米颗粒表面进行聚乙二醇化修饰以提高DNA复合物稳定性、将组织特异性配体加载在载体表面以提高转染的细胞靶向性(包括调节血管生成的内皮细胞)、加载特定化学键及使用可降解的生物材料以提高DNA与载体的解离速率等,从而促进非病毒载体有效递送基因[52]。除此之外,一些分子质量较大的阳离子聚合物虽然转染率高,但随之产生了过度的细胞毒性和血清成分干扰。 为了打破这种转染效率与安全性之间的相悖关系,近来的研究正不断通过降低分子质量并组合多种非病毒载体等方式增加载体的结构多样性,以构建综合性能优异的新型非病毒载体[53]。 病毒载体和非病毒载体性能特点,见表1。"
| [1] 俞舒舒,王彦林.先天性心脏病与新型环境因素相关性研究进展[J].中国计划生育学杂志,2019,27(6):823-826. [2] 陆铖,王江玥,贾仲林.影响非综合征型唇腭裂的环境因素[J].华西口腔医学杂志,2019,37(5):547-550. [3] 林恒娜,顾秀瑛,张思维,等.全球恶性肿瘤发病年龄分析[J].中华肿瘤杂志,2018,40(7):543-549. [4] SHAPIRO G, LIEBER R, GAZIT D, et al. Recent Advances and Future of Gene Therapy for Bone Regeneration. Curr Osteoporos Rep. 2018; 16(4):504-511. [5] BORRELLI MR, HU MS, LONGAKER MT, et al. Tissue Engineering and Regenerative Medicine in Craniofacial Reconstruction and Facial Aesthetics. J Craniofac Surg. 2020;31(1):15-27. [6] SMITH BT, SHUM J, WONG M, et al. Bone Tissue Engineering Challenges in Oral & Maxillofacial Surgery. Adv Exp Med Biol. 2015;881:57-78. [7] BHUMIRATANA S, BERNHARD JC, ALFI DM, et al. Tissue-engineered autologous grafts for facial bone reconstruction. Sci Transl Med. 2016; 8(343):343r-383r. [8] RADEMAKERS T, HORVATH JM, VAN BLITTERSWIJK CA, et al. Oxygen and nutrient delivery in tissue engineering: Approaches to graft vascularization. J Tissue Eng Regen Med. 2019;13(10):1815-1829. [9] SHARMA D, ROSS D, WANG G, et al. Upgrading prevascularization in tissue engineering: A review of strategies for promoting highly organized microvascular network formation. Acta Biomater. 2019;95: 112-130. [10] LIN Y, HUANG S, ZOU R, et al. Calcium phosphate cement scaffold with stem cell co-culture and prevascularization for dental and craniofacial bone tissue engineering. Dent Mater. 2019;35(7):1031-1041. [11] WANG L, SHI Q, CAI Y, et al. Mechanical-chemical coupled modeling of bone regeneration within a biodegradable polymer scaffold loaded with VEGF. Biomech Model Mechanobiol. 2020;19(6):2285-2306. [12] QUINLAN E, LOPEZ-NORIEGA A, THOMPSON EM, et al. Controlled release of vascular endothelial growth factor from spray-dried alginate microparticles in collagen-hydroxyapatite scaffolds for promoting vascularization and bone repair. J Tissue Eng Regen Med. 2017;11(4):1097-1109. [13] YIN S, ZHANG W, ZHANG Z, et al. Recent Advances in Scaffold Design and Material for Vascularized Tissue-Engineered Bone Regeneration. Adv Healthc Mater. 2019;8(10):e1801433. [14] Mitchell AC, Briquez PS, Hubbell JA, et al. Engineering growth factors for regenerative medicine applications. Acta Biomater. 2016;30:1-12. [15] BETZ VM, KOCHANEK S, RAMMELT S, et al. Recent advances in gene-enhanced bone tissue engineering. J Gene Med. 2018;20(6):e3018. [16] HASSANZADEH P, ATYABI F, DINARVAND R. Tissue engineering: Still facing a long way ahead. J Control Release. 2018;279:181-197. [17] ATASOY-ZEYBEK A, KOSE GT. Gene Therapy Strategies in Bone Tissue Engineering and Current Clinical Applications. Adv Exp Med Biol. 2018; 1119:85-101. [18] LEE HL, LEE HY, YUN Y, et al. Hypoxia-specific, VEGF-expressing neural stem cell therapy for safe and effective treatment of neuropathic pain. J Control Release. 2016;226:21-34. [19] 张志翔,徐恋祎.基因治疗用于骨组织工程的研究进展[J].临床口腔医学杂志,2020,36(3):180-184. [20] ZHA Y, LI Y, LIN T, et al. Progenitor cell-derived exosomes endowed with VEGF plasmids enhance osteogenic induction and vascular remodeling in large segmental bone defects. Theranostics. 2021;11(1):397-409. [21] WANG J, CHEN Z, SUN M, et al. Characterization and therapeutic applications of mesenchymal stem cells for regenerative medicine. Tissue Cell. 2020;64:101330. [22] TROHATOU O, ROUBELAKIS MG. Mesenchymal Stem/Stromal Cells in Regenerative Medicine: Past, Present, and Future. Cell Reprogram. 2017;19(4):217-224. [23] XIE Q, LIU R, JIANG J, et al. What is the impact of human umbilical cord mesenchymal stem cell transplantation on clinical treatment? Stem Cell Res Ther. 2020;11(1):519. [24] GARCIA-VAZQUEZ MD, HERRERO DLPB, GARCIA-ALONSO I, et al. Analysis of Biological Properties of Human Adult Mesenchymal Stem Cells and Their Effect on Mouse Hind Limb Ischemia. J Vasc Res. 2019; 56(2):77-91. [25] TAN S, TJIO C, WONG J, et al. Mesenchymal Stem Cell Exosomes for Cartilage Regeneration: A Systematic Review of Preclinical In Vivo Studies. Tissue Eng Part B Rev. 2021;27(1):1-13. [26] NOWAKOWSKI A, WALCZAK P, JANOWSKI M, et al. Genetic Engineering of Mesenchymal Stem Cells for Regenerative Medicine. Stem Cells Dev. 2015;24(19):2219-2242. [27] HUANG ZW, LIU N, LI D, et al. Angiopoietin-1 Modified Human Umbilical Cord Mesenchymal Stem Cell Therapy for Endotoxin-Induced Acute Lung Injury in Rats. Yonsei Med J. 2017;58(1):206-216. [28] ZHU Y, YI Y, YANG S, et al. Construction of injectable tissue engineered adipose tissue with fibrin glue scaffold and human adipose-derived stem cells transfected by lentivirus vector expressing hepatocyte growth factor. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2017;31(9): 1111-1118. [29] 蒋星海,赵彪,吴凯,等.血管内皮生长因子165、神经营养因子3、血管生成素1基因转染诱导骨髓间充质干细胞向神经元及血管内皮细胞分化[J].中国组织工程研究,2018,22(25):3956-3962. [30] ZHANG F, PENG WX, WANG L, et al. Role of FGF-2 Transfected Bone Marrow Mesenchymal Stem Cells in Engineered Bone Tissue for Repair of Avascular Necrosis of Femoral Head in Rabbits. Cell Physiol Biochem. 2018;48(2):773-784. [31] 陈波,郭祥,张寿,等.转染BMP-2基因的骨髓MSC复合多孔磷酸钙骨水泥构建组织工程化骨对兔骨缺损的修复作用研究[J].临床和实验医学杂志,2019,18(17):1815-1818. [32] KRUGER-GENGE A, BLOCKI A, FRANKE RP, et al. Vascular Endothelial Cell Biology: An Update. Int J Mol Sci. 2019;20(18):4411. [33] 明华伟,何芸,夏德林,等. VEGF 165基因转染HUVEC促进大鼠血管生成的实验研究[J].口腔颌面外科杂志,2017,27(3):161-165. [34] PETERS EB. Endothelial Progenitor Cells for the Vascularization of Engineered Tissues. Tissue Eng Part B Rev. 2018;24(1):1-24. [35] 王娟.VEGF基因修饰的内皮祖细胞治疗脑梗死的疗效和机制的实验研究[D].长春:吉林大学,2016. [36] 薛建新,秦志强,李潇,等.VEGF_(165)转染内皮祖细胞移植对大鼠肾脏缺血再灌注的保护作用[J]. 南京医科大学学报(自然科学版),2018,38(1):34-39. [37] 汪砚雨.VEGF165基因转染血管内皮祖细胞治疗心肌缺血再灌注损伤的实验研究[D].郑州:郑州大学,2018. [38] 李建君.VEGF-Ax对大鼠骨髓间充质干细胞成血管分化的调节作用及其促血管生成能力的研究[D]. 广州:南方医科大学,2020. [39] ALMUBARAK S, NETHERCOTT H, FREEBERG M, et al. Tissue engineering strategies for promoting vascularized bone regeneration. Bone. 2016; 83:197-209. [40] CAPORARELLO N, D’ANGELI F, CAMBRIA MT, et al. Pericytes in Microvessels: From “Mural” Function to Brain and Retina Regeneration. Int J Mol Sci. 2019;20(24):6351. [41] 余艳妮.装载经RGD修饰的VEGF165和Ang-1双基因共表达腺病毒载体的丝素多孔支架及其促血管化作用[D].苏州:苏州大学, 2015. [42] LI B, WANG H, QIU G, et al. Synergistic Effects of Vascular Endothelial Growth Factor on Bone Morphogenetic Proteins Induced Bone Formation In Vivo: Influencing Factors and Future Research Directions. Biomed Res Int. 2016;2016:2869572. [43] LEE E, KO JY, KIM J, et al. Osteogenesis and angiogenesis are simultaneously enhanced in BMP2-/VEGF-transfected adipose stem cells through activation of the YAP/TAZ signaling pathway. Biomater Sci. 2019;7(11):4588-4602. [44] EST-WITTE SE, FARRIS AL, TZENG SY, et al. Non-viral gene delivery of HIF-1alpha promotes angiogenesis in human adipose-derived stem cells. Acta Biomater. 2020;113:279-288. [45] YING C, WANG R, WANG Z, et al. BMSC-Exosomes Carry Mutant HIF-1alpha for Improving Angiogenesis and Osteogenesis in Critical-Sized Calvarial Defects. Front Bioeng Biotechnol. 2020;8:565561. [46] PENG WX, WANG L. Adenovirus-Mediated Expression of BMP-2 and BFGF in Bone Marrow Mesenchymal Stem Cells Combined with Demineralized Bone Matrix For Repair of Femoral Head Osteonecrosis in Beagle Dogs. Cell Physiol Biochem. 2017;43(4):1648-1662. [47] MAO Y, LIU X Q, SONG Y, et al. Fibroblast growth factor-2/platelet-derived growth factor enhances atherosclerotic plaque stability. J Cell Mol Med. 2020;24(1):1128-1140. [48] VIJAYAN A, A S, KUMAR GSV. PEG grafted chitosan scaffold for dual growth factor delivery for enhanced wound healing. Sci Rep. 2019;9(1): 19165. [49] LUKASHEV AN, ZAMYATNIN AJ. Viral Vectors for Gene Therapy: Current State and Clinical Perspectives. Biochemistry (Mosc). 2016;81(7):700-708. [50] DAVID RM, DOHERTY AT. Viral Vectors: The Road to Reducing Genotoxicity. Toxicol Sci. 2017;155(2):315-325. [51] PATIL S, GAO YG, LIN X, et al. The Development of Functional Non-Viral Vectors for Gene Delivery. Int J Mol Sci. 2019;20(21):5491. [52] KIM J, MIRANDO AC, POPEL AS, et al. Gene delivery nanoparticles to modulate angiogenesis. Adv Drug Deliv Rev. 2017;119:20-43. [53] QADIR A, GAO Y, SURYAJI P, et al. Non-Viral Delivery System and Targeted Bone Disease Therapy. Int J Mol Sci. 2019;20(3):565. [54] RAFTERY RM, MENCIA CI, CHEN G, et al. Translating the role of osteogenic-angiogenic coupling in bone formation: Highly efficient chitosan-pDNA activated scaffolds can accelerate bone regeneration in critical-sized bone defects. Biomaterials. 2017;149:116-127. [55] POURLAK T, POURLAK T, GHODRATI M, et al. Usage of stem cells in oral and maxillofacial region. J Stomatol Oral Maxillofac Surg. 2020:S2468-7855(20)30235-4. [56] 秦宇星,任前贵,沈佩锋.组织工程骨技术治疗骨缺损的优越性[J].中国组织工程研究,2020,24(24):3877-3882. [57] TIRPE AA, GULEI D, CIORTEA SM, et al. Hypoxia: Overview on Hypoxia-Mediated Mechanisms with a Focus on the Role of HIF Genes. Int J Mol Sci. 2019;20(24):6140. [58] OMORPHOS NP, GAO C, TAN SS, et al. Understanding angiogenesis and the role of angiogenic growth factors in the vascularisation of engineered tissues. Mol Biol Rep. 2021;48(1):941-950. [59] BAI Y, BAI L, ZHOU J, et al. Sequential delivery of VEGF, FGF-2 and PDGF from the polymeric system enhance HUVECs angiogenesis in vitro and CAM angiogenesis. Cell Immunol. 2018;323:19-32. [60] 曹俊霞,王友亮,王征旭.精准调控CRISPR/Cas9基因编辑技术研究进展[J].遗传,2020,42(12):1168-1177. [61] 公少华,李娜,唐波.调控CRISPR-Cas9系统用于基因编辑的研究进展[J].化学学报,2020,78(7):634-641. |
| [1] | Zhu Chan, Han Xuke, Yao Chengjiao, Zhou Qian, Zhang Qiang, Chen Qiu. Human salivary components and osteoporosis/osteopenia [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1439-1444. |
| [2] | Jin Tao, Liu Lin, Zhu Xiaoyan, Shi Yucong, Niu Jianxiong, Zhang Tongtong, Wu Shujin, Yang Qingshan. Osteoarthritis and mitochondrial abnormalities [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1452-1458. |
| [3] | Zhang Lichuang, Xu Hao, Ma Yinghui, Xiong Mengting, Han Haihui, Bao Jiamin, Zhai Weitao, Liang Qianqian. Mechanism and prospects of regulating lymphatic reflux function in the treatment of rheumatoid arthritis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1459-1466. |
| [4] | Li Huo, Wang Peng, Gao Jianming, Jiang Haoran, Lu Xiaobo, Peng Jiang. Relationship between revascularization and internal microstructure changes in osteonecrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1323-1328. |
| [5] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [6] | Wang Jing, Xiong Shan, Cao Jin, Feng Linwei, Wang Xin. Role and mechanism of interleukin-3 in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1260-1265. |
| [7] | Zhu Chan, Han Xuke, Yao Chengjiao, Zhang Qiang, Liu Jing, Shao Ming. Acupuncture for Parkinson’s disease: an insight into the action mechanism in animal experiments [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1272-1277. |
| [8] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [9] | Fan Yiming, Liu Fangyu, Zhang Hongyu, Li Shuai, Wang Yansong. Serial questions about endogenous neural stem cell response in the ependymal zone after spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1137-1142. |
| [10] | Hui Xiaoshan, Bai Jing, Zhou Siyuan, Wang Jie, Zhang Jinsheng, He Qingyong, Meng Peipei. Theoretical mechanism of traditional Chinese medicine theory on stem cell induced differentiation [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1125-1129. |
| [11] | Guo Jia, Ding Qionghua, Liu Ze, Lü Siyi, Zhou Quancheng, Gao Yuhua, Bai Chunyu. Biological characteristics and immunoregulation of exosomes derived from mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1093-1101. |
| [12] | Wu Weiyue, Guo Xiaodong, Bao Chongyun. Application of engineered exosomes in bone repair and regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1102-1106. |
| [13] | Zhou Hongqin, Wu Dandan, Yang Kun, Liu Qi. Exosomes that deliver specific miRNAs can regulate osteogenesis and promote angiogenesis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1107-1112. |
| [14] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [15] | Huang Chenwei, Fei Yankang, Zhu Mengmei, Li Penghao, Yu Bing. Important role of glutathione in stemness and regulation of stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1119-1124. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||