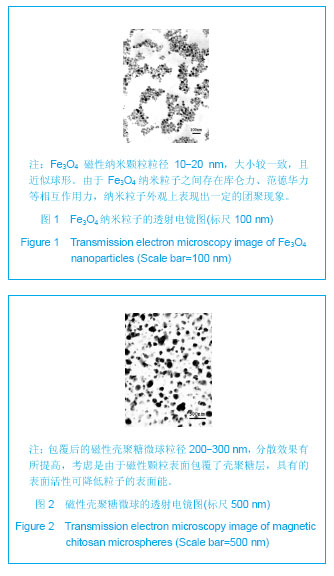
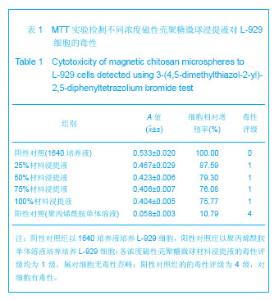
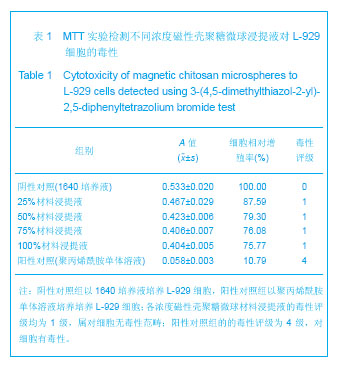
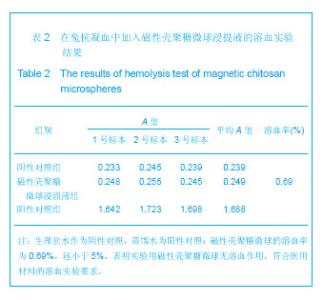
| [1] 李广峰,杨建东.壳聚糖纳米粒子基因载体的研究现状[J].中国组织工程研究与临床康复,2011,15(47):8879-8882.
[2] Lin YH,Mi FL,Chen CT,et al.Preparation and characterization of nanoparticles shelled with chitosan for oral insulin delivery. Biomacromolecules. 2007;8(1):146-152.
[3] 徐缨龙,蒋辉,汪景洲,等.制备肝靶向载体半乳糖基化壳聚糖/ DNA 纳米颗粒转染肝癌细胞SMMC- 7721 的体外实验[J].现代预防医学,2010,37(18):3507-3510.
[4] 陈道桢,李向东,唐秋莎,等. 超顺磁性氧化铁纳米粒子的制备、表征及生物相容性研究[J].山东医药,2012,52(39):1-4.
[5] 张皓,侯欣欣,张东生.磁性纳米材料在肿瘤热化疗中的研究进展[J].东南大学学报:医学版,2012,31(4):483-488.
[6] 杜益群,张东生,倪海燕,等. 肿瘤热疗用Fe3O4 磁性纳米粒的制备及表征[J].电子显微学报,2005,24(6):608-612.
[7] 葛玉卿,张宇,顾宁. 壳聚糖修饰氧化铁磁性纳米颗粒的制备和性能研究[J].功能材料与器件学报,2012,18(2):147-152.
[8] 季业,邵惠萍,郭志猛,等. 超声乳化法制备纳米Fe3O4磁性颗粒及壳聚糖表面改性[J].北京科技大学学报,2011,33(6):751-755.
[9] 周恒,罗聪,黄华. 医用磁性纳米粒的研究进展[J].中国医院药学杂志,2010,30(8):689-692.
[10] 陈朝婷,高峰,陆平,等.磁性壳聚糖纳米介导eNOS 基因转染受损平滑肌细胞初探[J].东南大学学报:医学版,2011,30(6): 827-831.
[11] 何荣芬,袁刚,邓旭晖. 磁性壳聚糖微球研究进展[J].检验医学与临床,2011,8(12):1500-1502.
[12] 颜士岩,张东生,顾宁,等.肿瘤热疗用Fe2O3 纳米磁性粒子的生物相容性研究[J].东南大学学报:医学版,2005,24(1):8-12.
[13] Wang ZY,Song J,Zhang DS.Nanosized As2O3/Fe2O3 complexes combined with magnetic fluid hyperthermia selectively target liver cancer cells. World J Gastroenterol. 2009;15(24):2995-3002.
[14] 杜益群,张东生,倪海燕,等.肿瘤热疗用Fe3O4 磁性纳米粒子的生物相容性研究[J].南京大学学报:自然科学版,2006,43(2): 324-330.
[15] 邹芬,潘一峰,张红,等. 聚乙二醇-聚乙烯亚胺/四氧化三铁纳米磁流体的毒性及其生物相容性[J].中国组织工程研究与临床康复, 2010,14(3):447-451.
[16] 吕晓迎,Kappert HF. 牙科材料细胞毒性评定的新方法(MTT 试验) [J].中华口腔医学杂志,1995,30(6):377-379.
[17] 刘静,张东生. 肿瘤热疗用纳米磁性材料的生物相容性评价方法研究进展[J]. 东南大学学报:医学版,2010,29(5):587-592.
[18] oxide nanoparticle aqueous suspensions to Zebrafish (Danio rerio) early developmental stage. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2008;43(3):278-284. |