Chinese Journal of Tissue Engineering Research ›› 2022, Vol. 26 ›› Issue (10): 1574-1581.doi: 10.12307/2022.206
Previous Articles Next Articles
Function on 3D printing poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/calcium sulfate hemihydrate scaffold integrated chitosan hydrogel coating
Ye Xiangling1, Xia Yuanjun2, Wang Boqun3, Kang Zhengyang4, Wu Bin4
- 1Fifth Clinical College of Guangzhou University of Chinese Medicine, Guangzhou 510405, Guangdong Province, China; 2Department of Trauma Orthopedics, General Hospital of Southern Theater Command of PLA, Guangzhou 510010, Guangdong Province, China
-
Received:2021-03-18Revised:2021-03-20Accepted:2021-05-17Online:2022-04-08Published:2021-10-27 -
Contact:Wu Bin, Associate chief physician, Department of Orthopedics, Second People’s Hospital of Guangzhou Panyu, Guangzhou 510160, Guangdong Province, China -
About author:Ye Xiangling, Doctoral candidate, Fifth Clinical College of Guangzhou University of Chinese Medicine, Guangzhou 510405, Guangdong Province, China -
Supported by:the Guangzhou Science and Technology Planning Project, No. 201804010136 (to XYJ)
CLC Number:
Cite this article
Ye Xiangling, Xia Yuanjun, Wang Boqun, Kang Zhengyang, Wu Bin. Function on 3D printing poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/calcium sulfate hemihydrate scaffold integrated chitosan hydrogel coating[J]. Chinese Journal of Tissue Engineering Research, 2022, 26(10): 1574-1581.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
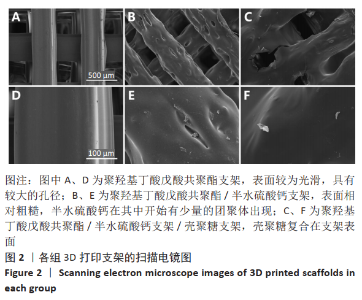
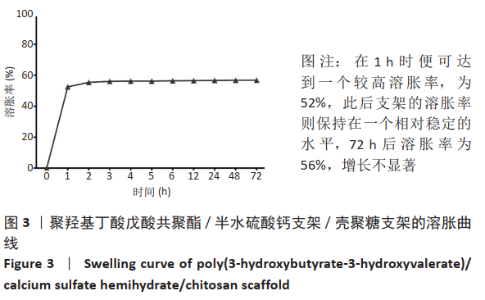
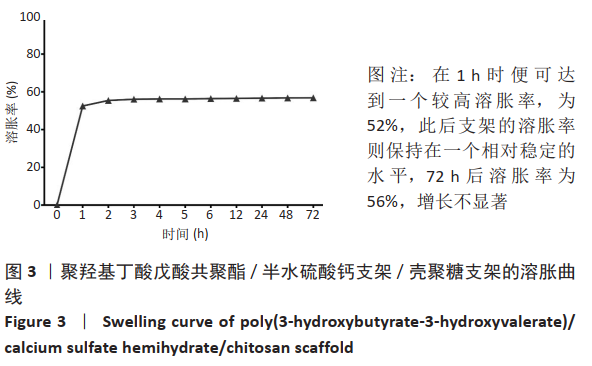
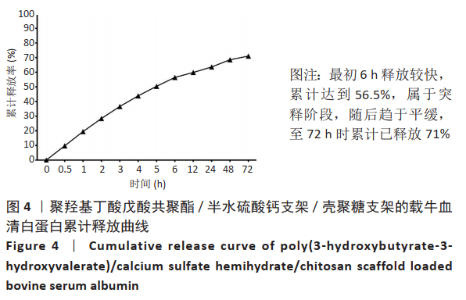
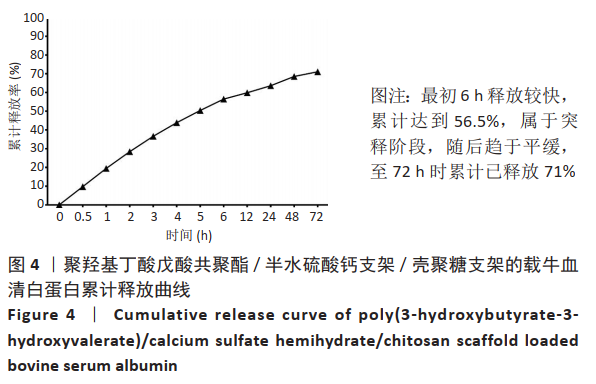
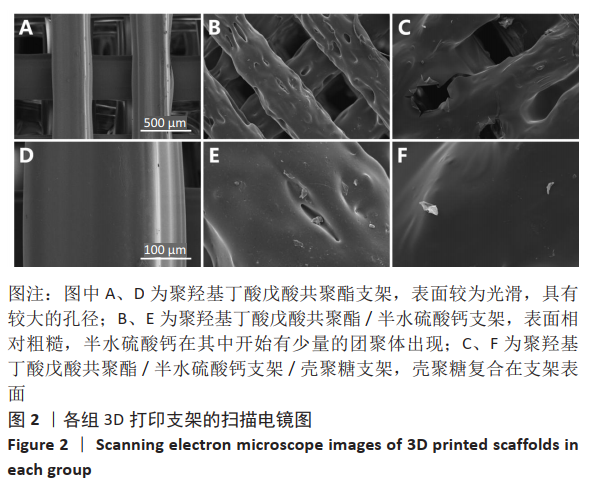
2.1 各组支架的表面形貌 使用纯聚羟基丁酸戊酸共聚酯所打印的支架表面较为光滑,并且支架本身具有较大的孔径,平均孔径达到了约400 μm,大孔经为水凝胶浸渍并形成相互连接的结构提供了足够的空间;聚羟基丁酸戊酸共聚酯/半水硫酸钙支架表面相对粗糙,且半水硫酸钙在其中开始有少量的团聚体出现;将聚羟基丁酸戊酸共聚酯/半水硫酸钙支架浸泡在壳聚糖溶液后,经过烘干,壳聚糖紧紧地复合在聚羟基丁酸戊酸共聚酯/半水硫酸钙支架表面,如图2所示。通过Micro-CT观察支架并对其进行三维重建后进行分析,可得通过计算得出聚羟基丁酸戊酸共聚酯支架的孔隙率为(59.25±2.68)%,连通性为(99.97±0.02)%,平均孔径为(400.65±38.58) μm。这说明支架具有较高的孔隙率、良好的孔隙分布及较大的孔径尺寸。"
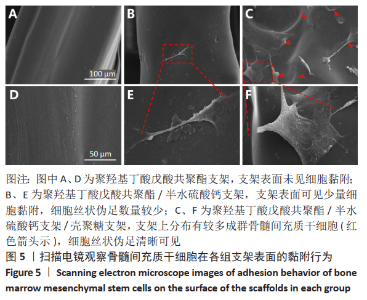
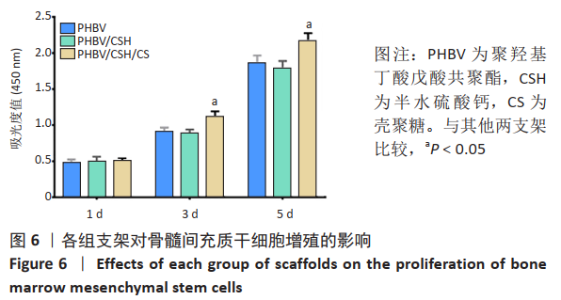
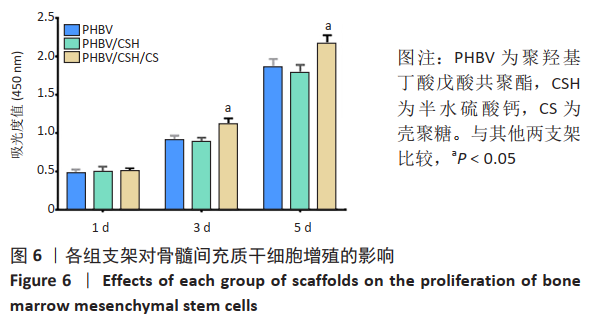
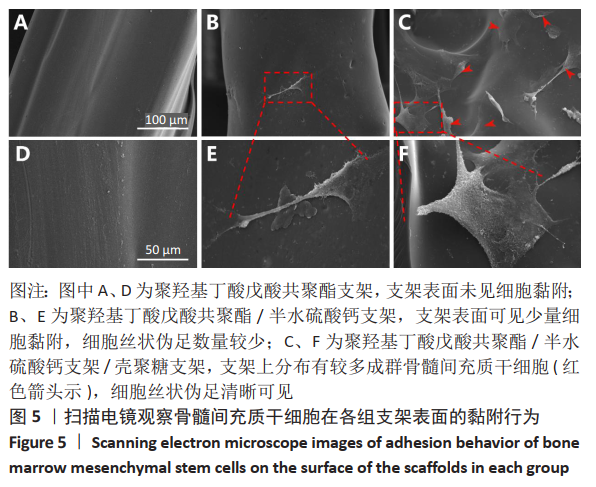
2.4 各组支架对细胞黏附行为的影响 见图5。 通过扫描电镜评估材料表面对细胞的黏附性能。如图5A显示,单纯的聚羟基丁酸戊酸共聚酯支架表面未见细胞黏附,这说明单纯的聚羟基丁酸戊酸共聚酯支架细胞黏附性能不佳。 图5B可见散落在聚羟基丁酸戊酸共聚酯/半水硫酸钙支架上的细胞。通过放大扫描电镜倍数,如图5E所示,骨髓间充质干细胞丝状伪足数量较少,并且没有完全伸展。图5C显示出PHBV/CSH/CS支架上分布有较多成群骨髓间充质干细胞(见图中红箭头所示),细胞呈梭形且伸展良好,细胞紧密黏附在材料表面。放大扫描电镜显示,图5F细胞丝状伪足清晰可见。这说明PHBV/CSH/CS支架不仅具有良好的生物相容性,而且显著提高支架表面的细胞黏附能力。"
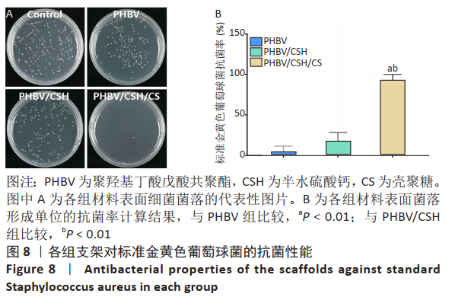
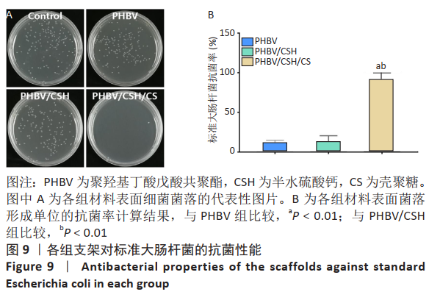
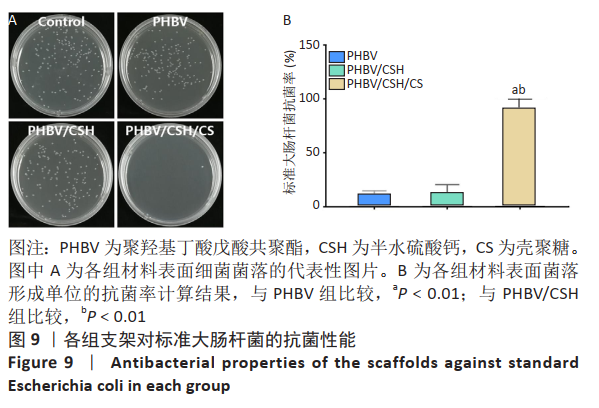
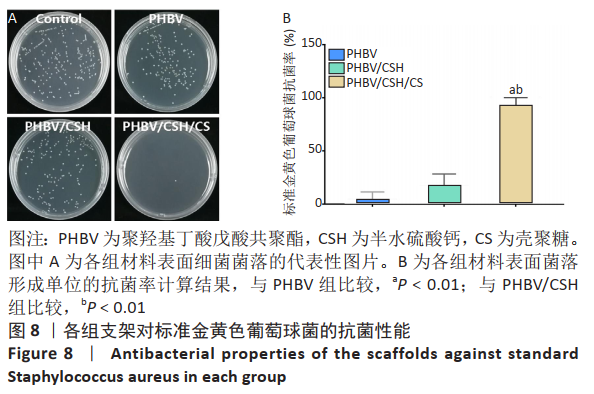
2.7 各组支架对标准金黄色葡萄球菌的抗菌性能 图8反映了各组支架对标准金黄色葡萄球菌的抗菌活性。聚羟基丁酸戊酸共聚酯、聚羟基丁酸戊酸共聚酯/半水硫酸钙支架和对照组之间没有明显差异(P > 0.05),这说明聚羟基丁酸戊酸共聚酯、聚羟基丁酸戊酸共聚酯/半水硫酸钙支架对标准金黄色葡萄球菌没有任何抗菌活性。而PHBV/CSH/CS支架抗菌能力显著高于聚羟基丁酸戊酸共聚酯、聚羟基丁酸戊酸共聚酯/半水硫酸钙支架(P < 0.01),通过计算表明,PHBV/CSH/CS支架对标准金黄色葡萄球菌的抗菌率达到(98.78±1.07)%,说明PHBV/CSH/CS支架对标准金黄色葡萄球菌表现出良好的抗菌活性。"
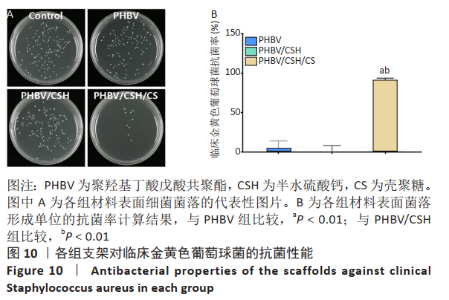
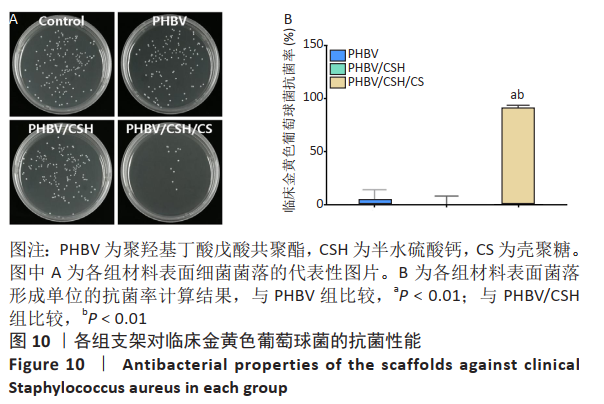
2.9 各组支架对临床金黄色葡萄球菌的抗菌性能 图10反映了各组支架对临床金黄色葡萄球菌的抗菌活性。聚羟基丁酸戊酸共聚酯、聚羟基丁酸戊酸共聚酯/半水硫酸钙支架和对照组之间没有明显差异(P > 0.05),这说明聚羟基丁酸戊酸共聚酯、聚羟基丁酸戊酸共聚酯/半水硫酸钙支架对临床金黄色葡萄球菌没有任何抗菌活性。PHBV/CSH/CS支架的抗菌能力显著高于聚羟基丁酸戊酸共聚酯、聚羟基丁酸戊酸共聚酯/半水硫酸钙支架(P < 0.01),通过计算表明,PHBV/CSH/CS对临床金黄色葡萄球菌的抗菌率达到(91.66±1.89)%,说明PHBV/CSH/CS支架对临床金黄色葡萄球菌表现出良好的抗菌活性。"
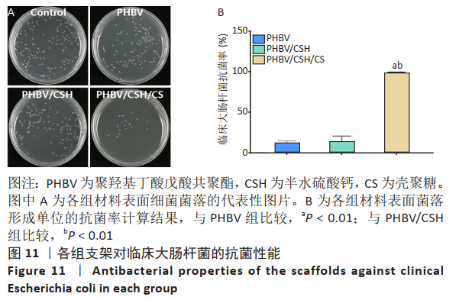
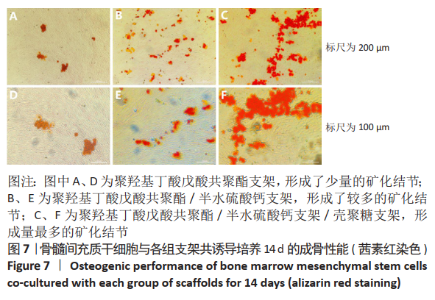
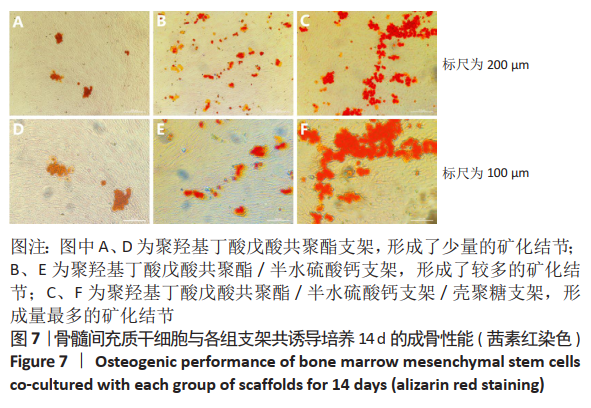
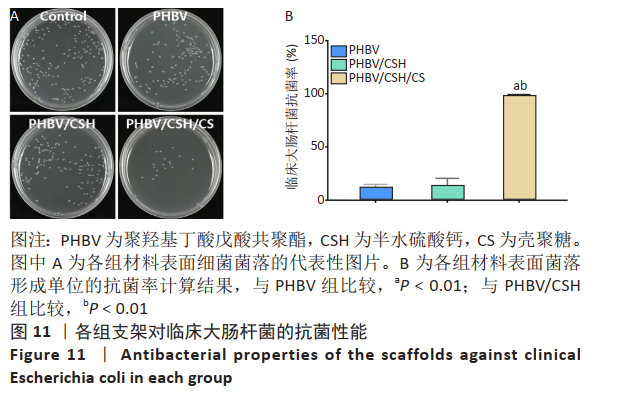
2.10 各组支架对临床大肠杆菌的抗菌性能 见图11。 图11反映了各组支架对临床大肠杆菌的抗菌活性。聚羟基丁酸戊酸共聚酯、聚羟基丁酸戊酸共聚酯/半水硫酸钙支架和对照组之间没有明显差异(P > 0.05),这说明聚羟基丁酸戊酸共聚酯、 聚羟基丁酸戊酸共聚酯/半水硫酸钙支架对临床大肠杆菌没有任何抗菌活性。PHBV/CSH/CS支架的抗菌能力显著高于聚羟基丁酸戊酸共聚酯、聚羟基丁酸戊酸共聚酯/半水硫酸钙支架(P < 0.01),通过计算表明,PHBV/CSH/CS支架对临床大肠杆菌的抗菌率达到(93.95±0.33)%,说明PHBV/CSH/CS支架对临床大肠杆菌表现出良好的抗菌活性。 2.11 支架的生物相容性 由细胞增殖与黏附实验可知,PHBV/CSH/CS支架具有良好的生物相容性。"
| [1] ALMUBARAK S, NETHERCOTT H, FREEBERG M, et al. Tissue engineering strategies for promoting vascularized bone regeneration. Bone. 2016;83: 197-209. [2] BANDYOPADHYAY A, MITRA I, BOSE S. 3D Printing for Bone Regeneration. Curr Osteoporos Rep. 2020;18(5):505-514. [3] CHEN Y, LI W, ZHANG C, et al. Recent Developments of Biomaterials for Additive Manufacturing of Bone Scaffolds. Adv Healthc Mater. 2020: e2000724. [4] KOVALCIK A, SANGRONIZ L, KALINA M, et al. Properties of scaffolds prepared by fused deposition modeling of poly(hydroxyalkanoates). Int J Biol Macromol. 2020;161:364-376. [5] CHERNOZEM RV, SURMENEVA MA, SHKARINA SN, et al. Piezoelectric 3-D Fibrous Poly(3-hydroxybutyrate)-Based Scaffolds Ultrasound-Mineralized with Calcium Carbonate for Bone Tissue Engineering: Inorganic Phase Formation, Osteoblast Cell Adhesion, and Proliferation. ACS Appl Mater Interfaces. 2019;11:19522-19533. [6] Goonoo N, Fahmi AW, Jonas U, et al. Improved Multicellular Response, Biomimetic Mineralization, Angiogenesis, and Reduced Foreign Body Response of Modified Polydioxanone Scaffolds for Skeletal Tissue Regeneration. ACS Appl Mater Interfaces. 2019;11: 5834-5850. [7] KOVALCIK A , SANGRONIZ L, KALINA M, et al. Properties of scaffolds prepared by fused deposition modeling of poly(hydroxyalkanoates). Int J Biol Macromol. 2020;161: 364-376. [8] BIAZAR E, KESHEL SH. Electrospun poly (3-hydroxybutyrate-co-3-hydroxyvalerate)/hydroxyapatite scaffold with unrestricted somatic stem cells for bone regeneration. Asaio J. 2015;61(3):357. [9] HOFFMAN AS. Hydrogels for biomedical applications. Ann N Y Acad Sci. 2001;944(1):62. [10] AUGST AD, KONG HJ, MOONEY DJ. Alginate hydrogels as biomaterials. Macromolecular Bioscience,2006;6(8):623-633. [11] DEMIRTAS TT, IRMAK G, GUMUSDERELIOGLU M. Bioprintable form of chitosan hydrogel for bone tissue engineering. Biofabrication. 2017;9(3): 35003. [12] NING C, QIAN J, LIU T, et al. Hyaluronic acid hydrogel scaffolds with a triple degradation behavior for bone tissue engineering. Carbohydr Polym. 2015; 126:192-198. [13] NOBLE L, GRAY AI, SADIQ L, et al. A non-covalently cross-linked chitosan based hydrogel. Int J Pharm. 1999;192(2):173-182. [14] REZAEI N, HAMIDABADI HG, KHOSRAVIMELAL S, et al. Antimicrobial peptides-loaded smart chitosan hydrogel: Release behavior and antibacterial potential against antibiotic resistant clinical isolates. Int J Biol Macromol. 2020;164:855-862. [15] WANG W, XUE C, MAO X. Chitosan: Structural modification, biological activity and application. Int J Biol Macromol. 2020;164:4532-4546. [16] KUMARI S, POTTATHARA YB, SADASIVUNI KK, et al. Surface functionalization of chitosan as a coating material for orthopaedic applications: A comprehensive review. Carbohydr Polym. 2021;255:117487. [17] HAMEDI H, MORADI S, HUDSON SM, et al. Chitosan based hydrogels and their applications for drug delivery in wound dressings: A review. Carbohydr Polym. 2018;199:445-460. [18] CAI T, WANG G, THOMPSON S, et al. Photonic Hydrogels with Poly (ethylene glycol) Derivative Colloidal Spheres as Building Blocks. Macromolecules. 2008;41(24):9508-9512. [19] 叶翔凌,章莹,夏远军,等.PHBV/CSH晶须复合材料的制备、表征及3D打印性能[J].塑料科技,2018,46(5):71-76. [20] LEE SS, KIM JH, JEONG J, et al. Sequential growth factor releasing double cryogel system for enhanced bone regeneration. Biomaterials. 2020;257: 120223. [21] AMIRYAGHOUBI N, FATHI M, PESYAN NN, et al. Bioactive polymeric scaffolds for osteogenic repair and bone regenerative medicine. Med Res Rev. 2020; 40:1833-1870. [22] ANTALYA H, JOHANNA B, RUSTOM LE, et al. Bone regeneration strategies: Engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials. 2018;180:143-162. [23] OVSIANIKOV A, KHADEMHOSSEINI A, MIRONOV V. The Synergy of Scaffold-Based and Scaffold-Free Tissue Engineering Strategies. Trends Biotechnol. 2018;36: 348-357. [24] BACKES EDUARDO H, FERNANDES EMANUEL M, DIOGO GABRIELA S, et al. Engineering 3D printed bioactive composite scaffolds based on the combination of aliphatic polyester and calcium phosphates for bone tissue regeneration. Mater Sci Eng C Mater Biol Appl. 2021;122:111928. [25] HAN X, SUN M, CHEN B, et al. Lotus seedpod-inspired internal vascularized 3D printed scaffold for bone tissue repair. Bioact Mater. 2021;6:1639-1652. [26] PEI X, WU L, ZHOU C, et al. 3D printed titanium scaffolds with homogeneous diamond-like structures mimicking that of the osteocyte microenvironment and its bone regeneration study. Biofabrication. 2020;13(1):015008. [27] MAHARJAN B, PARK J, KALIANNAGOUNDER VK, et al. Regenerated cellulose nanofiber reinforced chitosan hydrogel scaffolds for bone tissue engineering. Carbohydr Polym. 2021;251:117023. [28] ANISHA BS, SANKAR D, MOHANDAS A, et al. Chitosan-hyaluronan/nano chondroitin sulfate ternary composite sponges for medical use. Carbohydr Polym. 2013;92(2):1470-1476. [29] CHEN L, LIU J, GUAN M, et al. Growth Factor and Its Polymer Scaffold-Based Delivery System for Cartilage Tissue Engineering. Int J Nanomedicine. 2020;15:6097-6111. [30] XING H, LEE H, LUO L, et al. Extracellular matrix-derived biomaterials in engineering cell function. Biotechnol Adv. 2020;42:107421. [31] ANSELME K. Osteoblast adhesion on biomaterials. Bmaterials. 2000;21(7): 667-681. [32] SHUAI C, GUO W, GAO C, et al. Calcium Silicate Improved Bioactivity and Mechanical Properties of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Scaffolds. Polymers (Basel). 2017;9 (5): 175. [33] DU M, ZHU Y, YUAN L, et al. Assembled 3D cell niches in chitosan hydrogel network to mimic extracellular matrix. Colloid Surface A. 2013;434:78-87. [34] YUE S, HE H, LI B, et al. Hydrogel as a Biomaterial for Bone Tissue Engineering: A Review. Nanomaterials (Basel). 2020;10(8):1511. [35] HE Y, JIN Y, YING X, et al. Development of an antimicrobial peptide-loaded mineralized collagen bone scaffold for infective bone defect repair. Regen Biomater. 2020;7(5):515-525. [36] WEINSTEIN RA, DAROUICHE RO. Device-Associated Infections: A Macroproblem that Starts with Microadherence. Clin Infect Dis. 2001;33(9):1567-1572. [37] WHITEHOUSE JD, FRIEDMAN ND, KIRKLAND KB, et al. The Impact of Surgical-Site Infections Following Orthopedic Surgery at a Community Hospital and a University Hospital Adverse Quality of Life, Excess Length of Stay, and Extra Cost. Infect Control Hosp Epidemiol. 2002;23(4):183. [38] VERLEE A, MINCKE S, STEVENS CV. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr Polym. 2017;164:268-283. [39] 黄盛东,陈松,李思东.壳聚糖抗菌活性研究进展[J].广州化工,2014(24): 7-9. [40] ZHENG LY, ZHU JF. Study on antimicrobial activity of chitosan with different molecular weights. Carbohydr Polym. 2003;54(4):527-530. |
| [1] | Zhai Hongjie, Han Guanda, Li Lei, Dong Xiaohui, Jiang Zhiquan, Lou Feiyun. 3D printed polyetheretherketone material for skull defect repair [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(3): 380-384. |
| [2] | Li Rui, Liu Zhen, Guo Zige, Lu Ruijie, Wang Chen. Aspirin-loaded chitosan nanoparticles and polydopamine modified titanium sheets improve osteogenic differentiation [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(3): 374-379. |
| [3] | Li Yue, Lyu Yan, Feng Wanying, Song Yang, Yan Yu, Guan Yongge. Preparation of hyperoside nanoparticles to repair endometrial injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(3): 360-366. |
| [4] | Wu Lihao, Shao Anliang, Xu Lin, Ren Kang, Wang Hongjian, Chen Liang, Xu Ling. Evaluation of immunotoxicity of the absorbable macroporous polysaccharides composite hemostatic material [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(3): 329-334. |
| [5] | Jiang Haifang, Liu Rong, Hu Peng, Chen Wei, Wei Zairong, Yang Chenglan, Nie Kaiyu. Application of 3D printing technology in the precise and personalized treatment of cleft lip and palate [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(3): 413-419. |
| [6] | Song Yuxin, Zhang Tongtong, Niu Jianxiong, Wang Zengping, Wen Jie, Zhang Qunli, Xue Wen, Liu Lin. Precise screw placement of 3D printing model and orthopedic robot in spinal deformity [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(6): 904-907. |
| [7] | Wang Ruanbin, Cheng Liqian, Chen Kai. Application and value of polymer materials in three-dimensional printing biological bones and scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 610-616. |
| [8] | Zhang Tong, Cai Jinchi, Yuan Zhifa, Zhao Haiyan, Han Xingwen, Wang Wenji. Hyaluronic acid-based composite hydrogel in cartilage injury caused by osteoarthritis: application and mechanism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 617-625. |
| [9] | Huang Chuanjun, Zou Yu, Zhou Xiaoting, Zhu Yangqing, Qian Wei, Zhang Wei, Liu Xing. Transplantation of umbilical cord mesenchymal stem cells encapsulated in RADA16-BDNF hydrogel promotes neurological recovery in an intracerebral hemorrhage rat model [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 510-515. |
| [10] | Le Guoping, Zhang Ming, Xi Licheng, Luo Hanwen. Preparation and in vitro evaluation of vancomycin hydrochloride@polylactic acid-glycolic acid copolymer-chitosan-hyaluronic acid composite sustained-release microspheres [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 528-534. |
| [11] | He Guanyu, Xu Baoshan, Du Lilong, Zhang Tongxing, Huo Zhenxin, Shen Li. Biomimetic orientated microchannel annulus fibrosus scaffold constructed by silk fibroin [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 560-566. |
| [12] | Yang Yi, Cao Guangru, Wang Chong, Yuan Hao, Cai Yuqiang. 3D-printed thoracolumbar spine tuberculosis model and guide plate to guide the accuracy and safety of surgery [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(36): 5798-5806. |
| [13] | He Guowen, Hu Baijun, Gao Dawei, Chen Liang. Treatment of Schatzker type V and VI tibial plateau fractures with 3D printing preoperative planning combined with double reverse traction device [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(36): 5764-5769. |
| [14] | Long Zhisheng, Xiong Long, Gong Feipeng, Li Jingtang, Zeng Jianhua, Deng Ying, Lan Min, Kong Weihao, Chen Gang. Effect of artificial bone with multi-scale hydroxyapatite/chitosan microtubule structure on rabbit bone defect repair and angiogenesis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(34): 5436-5441. |
| [15] | Sun Xirao, Bao Jiaxin, Wang Chengyue. Construction of chitosan/mineralized collagen porous scaffold, osteogenic differentiation in vitro and biocompatibility [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(34): 5498-5503. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||