Chinese Journal of Tissue Engineering Research ›› 2022, Vol. 26 ›› Issue (4): 528-534.doi: 10.12307/2022.087
Previous Articles Next Articles
Preparation and in vitro evaluation of vancomycin hydrochloride@polylactic acid-glycolic acid copolymer-chitosan-hyaluronic acid composite sustained-release microspheres
Le Guoping, Zhang Ming, Xi Licheng, Luo Hanwen
- Department of Bone Disease and Joint Surgery/Bone Oncology, Fourth Affiliated Hospital of Guangxi Medical University, Liuzhou 545000, Guangxi Zhuang Autonomous Region, China
-
Received:2020-09-07Revised:2020-09-08Accepted:2020-10-24Online:2022-02-08Published:2021-11-03 -
Contact:Le Guoping, MD, Associate chief physician, Department of Bone Disease and Joint Surgery/Bone Oncology, Fourth Affiliated Hospital of Guangxi Medical University, Liuzhou 545000, Guangxi Zhuang Autonomous Region, China -
About author:Le Guoping, MD, Associate chief physician, Department of Bone Disease and Joint Surgery/Bone Oncology, Fourth Affiliated Hospital of Guangxi Medical University, Liuzhou 545000, Guangxi Zhuang Autonomous Region, China -
Supported by:Self-Funded Scientific Research Project of Guangxi Zhuang Autonomous Region Health Committee, No. Z20190244 (to LGP)
CLC Number:
Cite this article
Le Guoping, Zhang Ming, Xi Licheng, Luo Hanwen. Preparation and in vitro evaluation of vancomycin hydrochloride@polylactic acid-glycolic acid copolymer-chitosan-hyaluronic acid composite sustained-release microspheres[J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 528-534.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
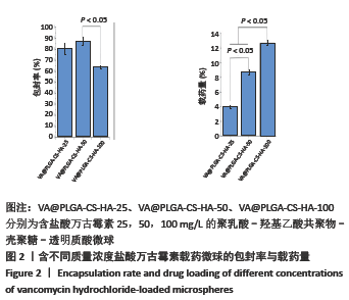
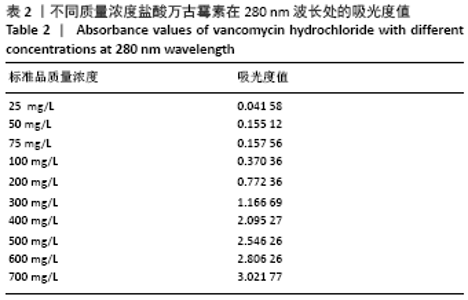
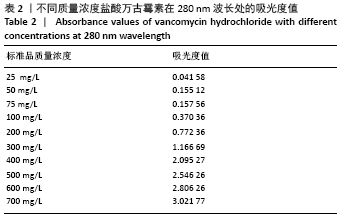
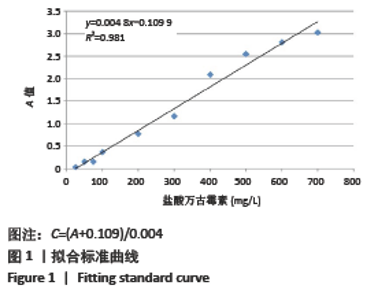
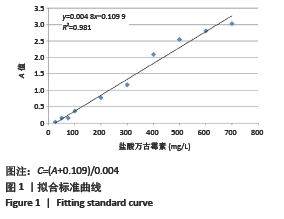
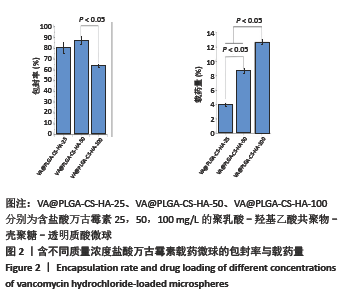
2.1.2 载药微球的包封率和载药量 3种微球的包封率和载药量,见图2。VA@PLGA-CS-HA-25微球的包封率为(79.70±5.11)%,载药量(3.98±0.26)%;VA@PLGA-CS-HA-50微球的包封率为(86.41±3.91)%,载药量为(8.64±0.39)%;VA@PLGA-CS-HA-100微球的包封率为(63.18±1.96)%,载药量为(12.63±0.39)%。 统计分析显示,VA@PLGA-CS-HA-50微球的包封率高于VA@PLGA-CS-HA-100微球(P < 0.05),VA@PLGA-CS-HA-25微球的包封率与VA@PLGA-CS-HA-100微球、VA@PLGA-CS-HA-50微球比较差异均无显著性意义(P > 0.05)。VA@PLGA-CS-HA-50微球的载药量高于VA@PLGA-CS-HA-25微球(P < 0.05),VA@PLGA-CS-HA-100微球的载药量高于VA@PLGA-CS-HA-50微球、VA@PLGA-CS-HA-25微球(P < 0.05)。"
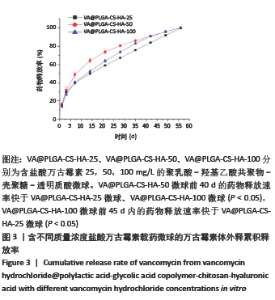
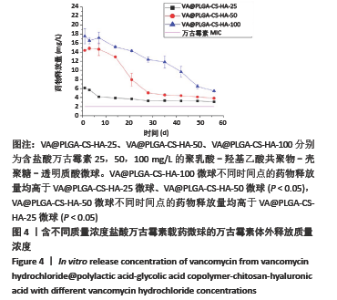
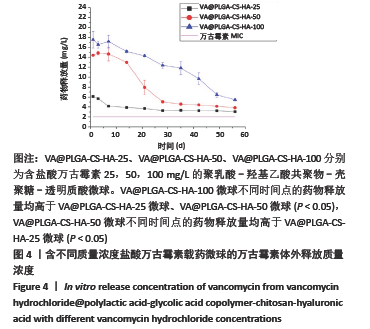
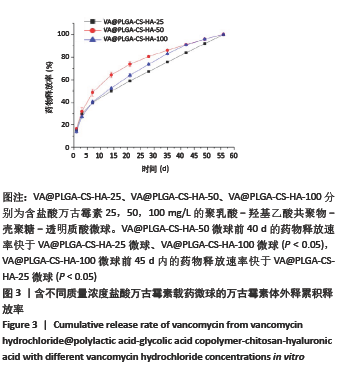
2.2 VA@PLGA-CS-HA微球的体外释放 3种微球24 h内未见明显突释现象,VA@PLGA-CS-HA-100、VA@PLGA-CS-HA-50、VA@PLGA-CS-HA-25微球24 h的累积释放率分别为(17.51±0.69)%,(13.78±0.96)%,(6.29±1.08)%。VA@PLGA-CS-HA-50微球所有时间点的药物释放速率都明显快于VA@PLGA-CS-HA-25微球和VA@PLGA-CS-HA-100微球,其中前40 d的药物释放速率比较差异有显著性意义(P < 0.05);VA@PLGA-CS-HA-100微球前45 d内的药物释放速率快于VA@PLGA-CS-HA-25微球(P < 0.05),见图3。VA@PLGA-CS-HA-100微球不同时间点的药物释放量均高于VA@PLGA-CS-HA-25微球、VA@PLGA-CS-HA-50微球(P < 0.05),VA@PLGA-CS-HA-50微球不同时间点的药物释放量均高于VA@PLGA-CS-HA-25微球(P < 0.05),见图4。"
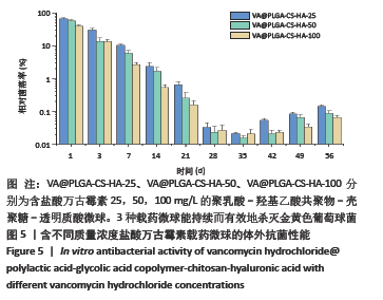
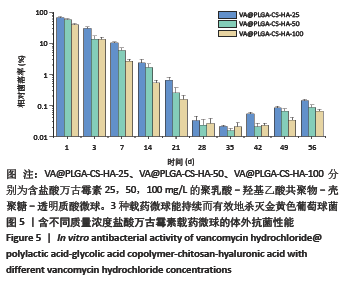
2.3 VA@PLGA-CS-HA微球的体外抗菌实验 图5为3种VA@PLGA-CS-HA微球的体外抗菌结果,可以看出在第1天,VA@PLGA-CS-HA-100微球的相对菌落率达30.58%,证明杀死了69%的金黄色葡萄球菌,而VA@PLGA-CS-HA-50微球和VA@ PLGA-CS-HA-25微球的相对菌落率分别为57.53%,65.68%,说明VA@PLGA-CS-HA-100微球的杀菌能力明显较其余两组高;到第7天时,VA@PLGA-CS-HA-100微球、VA@PLGA-CS-HA-50微球和VA@PLGA-CS-HA-25微球的相对菌落率分别为2.55%,7.35%,7.73%,均低于10%,说明3种含有盐酸万古霉素的抗菌缓释微球均能在一定时间内有效杀死金黄色葡萄球菌;在第14-28天期间,3种载药微球的相对菌落率低于3%,说明3种载药微球能持续而有效地杀灭金黄色葡萄球菌。"
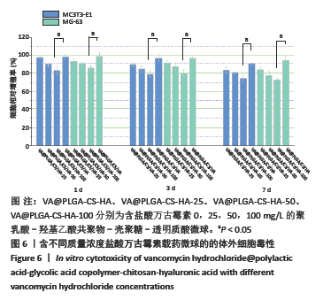
2.4 VA@PLGA-CS-HA微球的细胞毒性 图6为不同微球分别与MC3T3-E1细胞和MG-63细胞培养1,3,7 d之后的细胞毒性结果,可以明显地看出,VA@PLGA-CS-HA微球对两种细胞均没有细胞毒性,两种细胞的细胞相对增殖率均在90%以上;而VA@PLGA-CS-HA微球的细胞相对增殖率有明显的下降,VA@PLGA-CS-HA-25微球和VA@PLGA-CS-HA-50微球在1,3,7 d两种细胞的细胞相对增长率均在80%以上,证明两种微球没有明显的细胞毒性;VA@PLGA-CS-HA-100微球在第3天开始对两种细胞有轻微的细胞毒性细胞(细胞相对增殖率75%-82%),到第7天细胞毒性相对明显(细胞相对增殖率70%-78%)以上,证明两种微球没有明显的细胞毒性;VA@PLGA-CS-HA-100微球在第3天开始对两种细胞有轻微的细胞毒性细胞(细胞相对增殖率75%-82%),到第7天细胞毒性相对明显(细胞相对增殖率70%-78%)。 2.5 载药微球的生物相容性 由体外细胞毒性实验检测结果可知,含盐酸万古霉素25,50 g/L载药微球对MC3T3-E1细胞和MG-63细胞无明显的细胞毒性,100 g/L载药微球具有一定的细胞毒性。"
| [1] CHEN AT, VALLIER HA. Noncontiguous and open fractures of the lower extremity: epidemiology, complications, and unplanned procedures. Injury. 2016;47(3):742-747. [2] ROUSSIGNOL X, SIGONNEY G, POTAGE D, et al. Secondary nailing after external fixation for tibial shaft fracture: risk factors for union and infection. A 55 case series. Orthop Traumatol Surg Res. 2015; 101(1):89-92. [3] BIDAULT P, CHANDAD F, GRENIER D. Risk of bacterial resistance associated with systemic antibiotic therapy in periodontology. J Can Dent Assoc. 2007;73(8):721-725. [4] WALENKAMP GH, VREE TB, VAN RENS TJ. Gentamicin-PMMA beads. Pharmacokinetic and nephrotoxicological study. Clin Orthop Relat Res. 1986;(205):171-183. [5] KUSHAL M, MONALI M, DURGAVATI M. Oral controlled release drug delivery system: an overview. Int Res J Pharm. 2013;4(3):6-15. [6] SAINI RK, BAGRI LP, BAJPAI AK. Nano-silver hydroxyapatite based antibacterial 3D scaffolds of gelatin/alginate/poly (vinyl alcohol) for bone tissue engineering applications. Colloids Surf B Biointerfaces. 2019;177:211-218. [7] 魏丽艳,刘畅,卞婧,等.北京积水潭医院创伤性骨髓炎患者病原菌分布及抗菌药物应用情况分析[J].临床药物治疗杂志,2019, 17(10):42-45. [8] UNAGOLLA JM, JAYASURIYA AC. Drug transport mechanisms and in vitro release kinetics of vancomycin encapsulated chitosan-alginate polyelectrolyte microparticles as a controlled drug delivery system. Eur J Pharm Sci. 2018;114:199-209. [9] CHEN T, AO N, HUANG H. Preparation and characterization of alginate/HACC/oyster shell powder biocomposite scaffolds for potential bone tissue engineering applications. RSC Adv. 2016; 6(42):35577-35588. [10] UENG SW, LIN SS, WANG IC, et al. Efficacy of vancomycin-releasing biodegradable poly(lactide-co-glycolide) antibiotics beads for treatment of experimental bone infection due to Staphylococcus aureus. J Orthop Surg Res. 2016;11(1):52. [11] 方洁,刘利艳,黄洁,等.优化万古霉素给药方案的研究[J].药学服务与研究,2017,17(3):179-182. [12] HARRINGTON S, OTT L, KARANU F, et al. A Versatile Microencapsulation Platform for Hyaluronic Acid and Polyethylene Glycol. Tissue Eng Part A. 2020.doi: 10.1089/ten.TEA.2019.0286. [13] NAJBERG M, MANSOR MH, TAILLÉ T, et al. Aerogel sponges of silk fibroin, hyaluronic acid and heparin for soft tissue engineering: Composition-properties relationship. Carbohydr Polym. 2020;237: 116107. [14] YUAN Y, SHI X, GAN Z, et al. Modification of porous PLGA microspheres by poly-l-lysine for use as tissue engineering scaffolds. Colloids Surf B Biointerfaces. 2018;161:162-168. [15] JIANG Z, DONG X, SUN Y. Charge effects of self-assembled chitosan-hyaluronic acid nanoparticles on inhibiting amyloid β-protein aggregation. Carbohydr Res. 2018;461:11-18. [16] 吕婷婷,关于丹参素钠-PLGA缓释微球的制备及药剂学性能评价[J].中国社区医师,2019,35(21):8-10. [17] KUSHWAH V, LOPES DG, KOUTSAMANIS I, et al. Evolution of the microstructure and the drug release upon annealing the drug loaded lipid-surfactant microspheres. Eur J Pharm Sci. 2020;147:105278. [18] 刘炜,刘菊,黄义新.万古霉素壳聚糖缓释微球的制备及其体外释药特性和抑菌作用研究[J].中国药房,2016,27(31):4443-4445. [19] SONG J, FENG H, WU M, et al. Preparation and characterization of arginine-modified chitosan/hydroxypropyl methylcellose antibacterial film. Int J Biol Macromol. 2020;145:750-758. [20] ARIMURA Y, YANO T, HIRANO M. Mitochondrial superoxide production contributes to vancomycin-induced renal tubular cell apoptosis. Free Radic Biol Med. 2012;52(9):1865-1873. [21] YOERUEK E, SPITZER MS, SAYGILI O, et al. Comparison of in vitro safety profiles of vancomycin and cefuroxime on human corneal endothelial cells for intracameral use. J Cataract Refract Surg. 2008;34(12):2139-2145. [22] 郭忠尚,毕龙,杨昊.万古霉素/磷酸钙骨水泥复合材料的细胞毒性研究[J].现代生物医学进展,2015,15(20):3813-3814. [23] IGARTUA M, HERNANDEZ RM, ESQUISABEL A. Stability of BSA encapsulated into PLGA microspheres using PAGE and capillary electrophoresis. Int J Pharmaceutics. 1998;169(1):45-54. [24] 刘亚珍,邱晓明,李松凯.正交设计优化万古霉素/聚乳酸-羟基乙酸共聚物微球的制备及体外药物释放[J].中国组织工程研究, 2019,23(2):211-217. [25] XIE X, LIN W, XING C. In vitro and in vivo evaluations of PLGA microspheres containing nalmefene. PloS One. 2015;10(5):e0125953. [26] 王小雨.结核分枝杆菌毒素Rv2872促进重组菌耐受万古霉素和影响生物膜形成的分子机理[D].重庆:西南大学,2018. [27] GAO C, DAI Y, CHANG W, et al. VraSR has an important role in immune evasion of Staphylococcus aureus with low level vancomycin resistance. Microbes Infect. 2019;21(8-9):361-367. [28] CEVHER E, ORHAN Z, MÜLAZIMOĞLU L, et al. Characterization of biodegradable chitosan microspheres containing vancomycin and treatment of experimental osteomyelitis caused by methicillin-resistant Staphylococcus aureus with prepared microspheres. Int J Pharm. 2006;317(2):127-135. [29] 王青香.伤口分泌物菌株鉴定联合药敏试验对慢性化脓性骨髓炎患者抗生素合理使用的影响[J].北方药学,2019,16(5):155-156. |
| [1] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [2] | Jiang Huanchang, Zhang Zhaofei, Liang De, Jiang Xiaobing, Yang Xiaodong, Liu Zhixiang. Comparison of advantages between unilateral multidirectional curved and straight vertebroplasty in the treatment of thoracolumbar osteoporotic vertebral compression fracture [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1407-1411. |
| [3] | Xue Yadong, Zhou Xinshe, Pei Lijia, Meng Fanyu, Li Jian, Wang Jinzi . Reconstruction of Paprosky III type acetabular defect by autogenous iliac bone block combined with titanium plate: providing a strong initial fixation for the prosthesis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1424-1428. |
| [4] | Li Wei, Zhu Hanmin, Wang Xin, Gao Xue, Cui Jing, Liu Yuxin, Huang Shuming. Effect of Zuogui Wan on bone morphogenetic protein 2 signaling pathway in ovariectomized osteoporosis mice [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1173-1179. |
| [5] | Wang Jing, Xiong Shan, Cao Jin, Feng Linwei, Wang Xin. Role and mechanism of interleukin-3 in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1260-1265. |
| [6] | Xiao Hao, Liu Jing, Zhou Jun. Research progress of pulsed electromagnetic field in the treatment of postmenopausal osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1266-1271. |
| [7] | Wu Bingshuang, Wang Zhi, Tang Yi, Tang Xiaoyu, Li Qi. Anterior cruciate ligament reconstruction: from enthesis to tendon-to-bone healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1293-1298. |
| [8] | Tian Chuan, Zhu Xiangqing, Yang Zailing, Yan Donghai, Li Ye, Wang Yanying, Yang Yukun, He Jie, Lü Guanke, Cai Xuemin, Shu Liping, He Zhixu, Pan Xinghua. Bone marrow mesenchymal stem cells regulate ovarian aging in macaques [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 985-991. |
| [9] | Hu Wei, Xie Xingqi, Tu Guanjun. Exosomes derived from bone marrow mesenchymal stem cells improve the integrity of the blood-spinal cord barrier after spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 992-998. |
| [10] | Gao Yujin, Peng Shuanglin, Ma Zhichao, Lu Shi, Cao Huayue, Wang Lang, Xiao Jingang. Osteogenic ability of adipose stem cells in diabetic osteoporosis mice [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 999-1004. |
| [11] | Hou Jingying, Guo Tianzhu, Yu Menglei, Long Huibao, Wu Hao. Hypoxia preconditioning targets and downregulates miR-195 and promotes bone marrow mesenchymal stem cell survival and pro-angiogenic potential by activating MALAT1 [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1005-1011. |
| [12] | Liang Xuezhen, Yang Xi, Li Jiacheng, Luo Di, Xu Bo, Li Gang. Bushen Huoxue capsule regulates osteogenic and adipogenic differentiation of rat bone marrow mesenchymal stem cells via Hedgehog signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1020-1026. |
| [13] | Cui Xing, Sun Xiaoqi, Zheng Wei, Ma Dexin. Huangqin Decoction regulates autophagy to intervene with intestinal acute graft-versus-host disease in mice [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1057-1062. |
| [14] | Wu Weiyue, Guo Xiaodong, Bao Chongyun. Application of engineered exosomes in bone repair and regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1102-1106. |
| [15] | Zhou Hongqin, Wu Dandan, Yang Kun, Liu Qi. Exosomes that deliver specific miRNAs can regulate osteogenesis and promote angiogenesis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1107-1112. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||