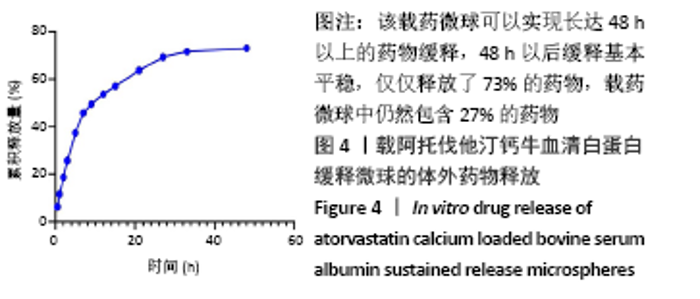
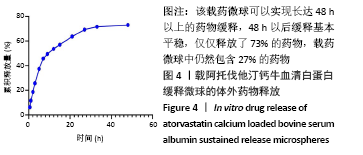
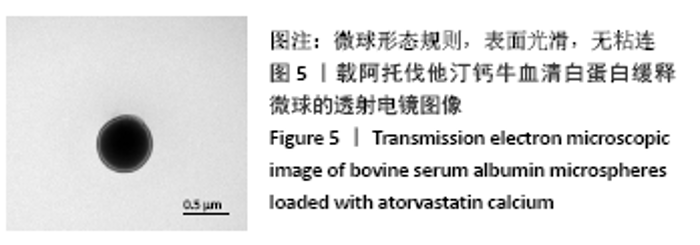
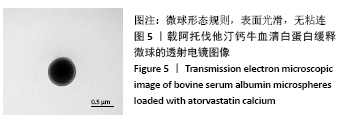
[1] TAN J, YANG N, FU X, et al. Single-Dose Local Simvastatin Injection Improves Implant Fixation via Increased Angiogenesis and Bone Formation in an Ovariectomized Rat Model. Med Sci Monit. 2015;21:1428-1439.
[2] SRIPRIYALAKSHMI S, ANJALI HC, C GEORGE PDC, et al. BSA Nanoparticle Loaded Atorvastatin Calcium - A New Facet for an Old Drug. PloS One. 2014;9(2):e86317.
[3] AN T, HAO J, SUN S, et al. Efficacy of statins for osteoporosis: a systematic review and meta-analysis. Osteoporos Int. 2017;28(1):47-57.
[4] 谢海风.阿托伐他汀联合降钙素对绝经期女性骨质疏松患者骨代谢指标的影响[J].中国妇幼保健,2020,35(3):495-497.
[5] 刘淑平,朱晓红,梁菊红,等.阿托伐他汀钙与低剂量雌激素联合治疗围绝经期女性骨质疏松的临床效果[J].贵州医药,2019,43(3): 395-396.
[6] 陈卫平.阿仑膦酸钠联合阿托伐他汀钙片治疗老年骨质疏松临床效果[J].中国合理用药探索,2020,17(5):61-66.
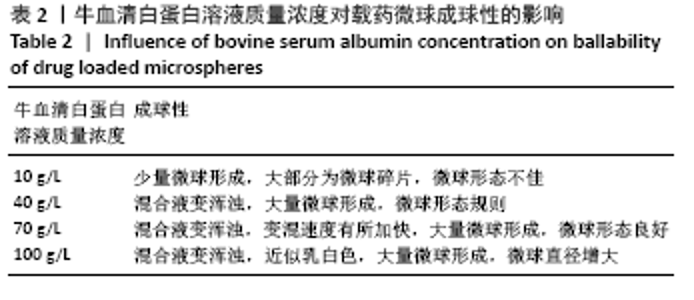
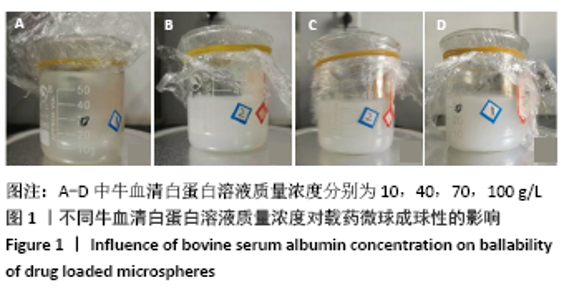
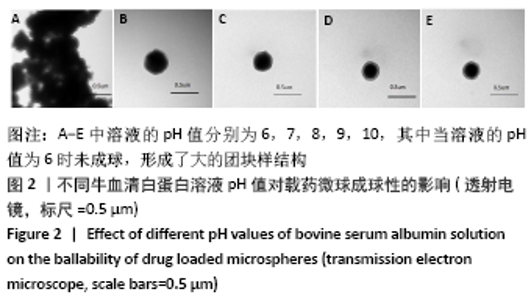
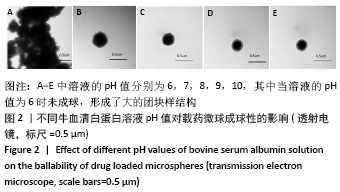
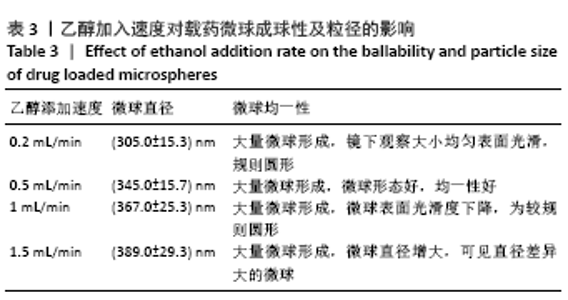
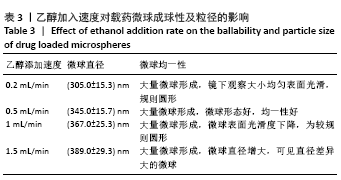
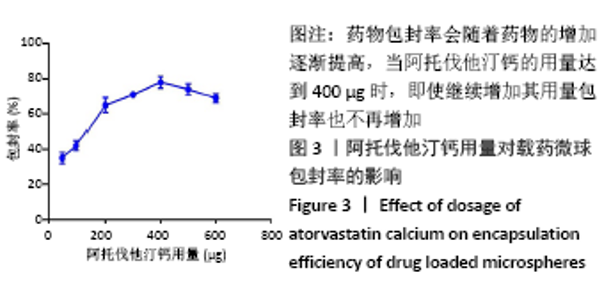
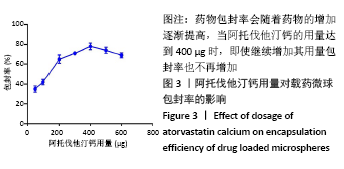
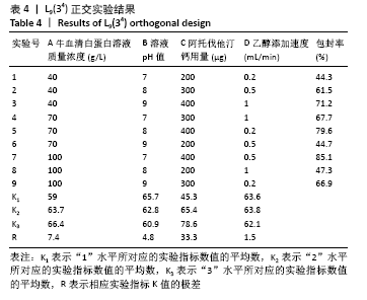
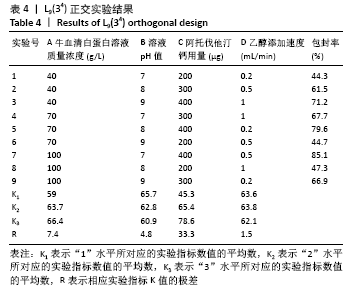
[7] 梁雪莉,朱秋兰,刘嫦玉,等.载牛血清白蛋白壳聚糖微球的制备与检测[J].华夏医学,2018,31(1):42-46.
[8] 李思阳,朱吉人,孔庆新,等.载牛血清白蛋白壳聚糖微球的制备及其体外释药特性评价[J].食品工业科技,2016,37(17):157-161.
[9] TIAN X, YIN H, ZHANG S, et al. Bufalin loaded biotinylated chitosan nanoparticles: An efficient drug delivery system for targeted chemotherapy against breast carcinoma. Eur J Pharm Biopharm. 2014; 87(3):445-453.
[10] LI FQ, SU H, WANG J, et al. Preparation and characterization of sodium ferulate entrapped bovine serum albumin nanoparticles for liver targeting. Int J Pharm. 2008;349(1):274-282.
[11] PAIK S, NGUYEN H H, RYU J, et al. Robust size control of bovine serum albumin (BSA) nanoparticles by intermittent addition of a desolvating agent and the particle formation mechanism. Food Chemistry. 2013;141(2):695-701.
[12] XU F, DING H, SONG F, et al. Effects of preparation methods on the bone formation potential of apatite-coated chitosan microspheres. J Biomater Sci Polym Ed. 2014;25(18):2080-2093.
[13] WU C, ZREIQAT H. Porous bioactive diopside (CaMgSi2O6) ceramic microspheres for drug delivery. Acta Biomater. 2010;6(3):820-829.
[14] WANG H, LEEUWENBURGH SCG, LI Y, et al. The Use of Micro- and Nanospheres as Functional Components for Bone Tissue Regeneration. Tissue Eng Part B Rev. 2012;18(1):24-39.
[15] LI Y, SONG H, XIONG S, et al. Chitosan-stablized bovine serum albumin nanoparticles having ability to control the release of NELL-1 protein. Int J Biol Macromol. 2018;109:672-680.
[16] JAHANBAN-ESFAHLAN A, PANAHI-AZAR V, SAJEDI S. Spectroscopic and molecular docking studies on the interaction between N-acetyl cysteine and bovine serum albumin. Biopolymers. 2015;103(11):638-645.
[17] JAHANBAN-ESFAHLAN A, PANAHI-AZAR V. Interaction of glutathione with bovine serum albumin: Spectroscopy and molecular docking. Food Chem. 2016;202(1):426-431. |