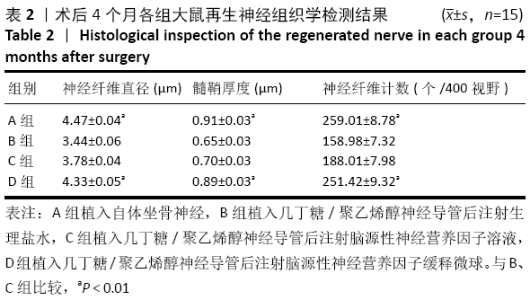
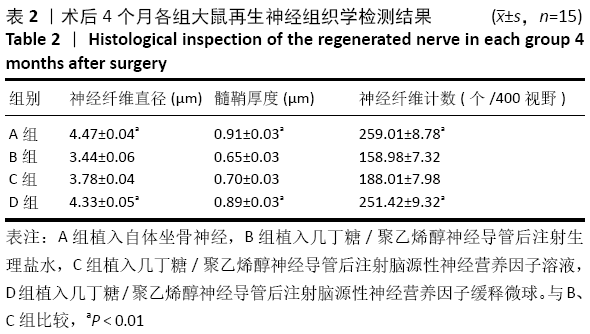
[1] 钱运,范存义.石墨烯促进周围神经再生的研究进展[J].中华手外科杂志,2019,35(2):158-160.
[2] 全琦,苌彪,刘若西,等.周围神经损伤与再生:新型修补材料的应用研究与进展[J].中国组织工程研究,2017,21(6):962-968.
[3] KONOFAOS P, VER HALEN JP. Nerve repair by means of tubulization: past,present, future. J Reconstr Microsurg.2013;29(3):149-164.
[4] LIN MY, MANZANO G, GUPTA R. Nerve allografts and conduits in peripheral nerve repair. Hand Clin.2013;29(3):331-348.
[5] KOWIANSKI P, LIETZAU G, CZUBA E, et al. BDNF: A Key Factor with Multipotent Impact on Brain Signaling and Synaptic Plasticity.Cell Mol Neurobiol.2018;38(3):579-593.
[6] 陈保国,黄渭清.脑源性生长因子与神经损伤的修复及再生[J].中国组织工程研究与临床康复,2010,14(15):2805-2809.
[7] 李勋,韦祎,马光辉,等.缓释微球制剂的研究进展[J].北京化工大学学报(自然科学版), 2017,44(6):1-11.
[8] 刘华蔚,温伟生,孙华燕,等.聚合物合金法制备神经生长因子缓释微球及其性能检测[J]. 中华临床医师杂志(电子版),2012,6(17): 5166-5170.
[9] 柯于海,张振伟,冯登殿,等.不同时段应用他克莫司纳米微球缓释颗粒促进同种异体神经移植再生的实验研究[J].中华手外科杂志,2010,26(4):230-233.
[10] 黄永旺,范学政.几丁糖与聚乳酸合成生物材料修复周围神经缺损[J].中国组织工程研究,2015,19(25):4059-4063.
[11] ZHAO Q, LI ZY, ZHANG ZP, et al. Polylactic-co-glycolic acid microspheres containing three neurotrophic factors promote sciatic nerve repair after injury. Neural Regen Res.2015;10(9):1491-1497.
[12] ZHAO HY, WU J, ZHU JJ, et al. Research Advances in Tissue Engineering Materials for Sustained Release of Growth Factors.Biomed Res Int.2015; 2015:808202.
[13] 蒋良福,劳杰,何继银,等.几丁糖胶原复合膜及激活态雪旺细胞修复周围神经缺损的实验研究[J].中华手外科杂志,2005,21(3): 172-174.
[14] 刘勇,侯春林,林浩东,等.几丁糖/聚乙烯醇神经导管修复猕猴周围神经缺损的实验研究[J].中国修复重建外科杂志,2011,25(10): 1235-1238.
[15] 刘勇,侯春林,林浩东,等.几丁糖复合聚乙烯醇神经导管修复大鼠坐骨神经损[J].中华显微外科杂志,2011,34(4):297-300.
[16] FEI J, GAO L, LI HH, et al. Electroacupuncture promotes peripheral nerve regeneration after facial nerve crush injury and upregulates the expression of glial cell-derived neurotrophic factor. Neural Regen Res. 2019;14(4):673-682.
[17] GIBON J, BARKER PA. Neurotrophins and Proneurotrophins:Focus on Synaptic Activity and Plasticity in the Brain.Neuroscientist.2017;23(6): 587-604.
[18] HEMPSTEAD BL. Deciphering proneurotrophin actions.Handb Exp Pharmacol.2014;220:17-32.
[19] MOHTARAM NK, MONTGOMERY A, WILLERTH SM. Biomaterial-based drug delivery systems for the controlled release of neurotrophic factors.Biomed Mater.2013;8(2):022001.
[20] MA F, WANG F, LI R, et al. Application of drug delivery systems for the controlled delivery of growth factors to treat nervous system injury.Organogenesis.2018;14(3):123-128.
[21] SANTOS D, GIUDETTI G, MICERA S, et al. Focal release of neurotrophic factors by biodegradable microspheres enhance motor and sensory axonal regeneration in vitro and in vivo.Brain Res.2016;1636:93-106.
[22] ZHANG XY, LIU F, CHEN Y, et al. Proprotein convertase 1/3-mediated down-regulation of brain-derived neurotrophic factor in cortical neurons induced by oxygen-glucose deprivation. Neural Regen Res. 2020;15(6):1066-1070.
|