Chinese Journal of Tissue Engineering Research ›› 2021, Vol. 25 ›› Issue (7): 1116-1121.doi: 10.3969/j.issn.2095-4344.2179
Previous Articles Next Articles
Photoreceptor cell replacement therapy for retinal degeneration diseases
Li Shanshan, Guo Xiaoxiao, You Ran, Yang Xiufen, Zhao Lu, Chen Xi, Wang Yanling
- Department of Ophthalmology, Beijing Friendship Hospital, Capital Medical University, Beijing 100050, China
-
Received:2019-09-30Revised:2019-10-21Accepted:2020-03-18Online:2021-03-08Published:2020-12-09 -
Contact:Wang Yanling, PhD, Chief physician, Department of Ophthalmology, Beijing Friendship Hospital, Capital Medical University, Beijing 100050, China Chen Xi, PhD, Department of Ophthalmology, Beijing Friendship Hospital, Capital Medical University, Beijing 100050, China -
About author:Li Shanshan, Doctoral candidate, Physician, Department of Ophthalmology, Beijing Friendship Hospital, Capital Medical University, Beijing 100050, China -
Supported by:the National Natural Science Foundation of China, No. 81870686; the Beijing Municipal Natural Science Foundation, No. 7184201; the Capital’s Funds for Health Improvement and Research (SF 2018-1-2021)
CLC Number:
Cite this article
Li Shanshan, Guo Xiaoxiao, You Ran, Yang Xiufen, Zhao Lu, Chen Xi, Wang Yanling. Photoreceptor cell replacement therapy for retinal degeneration diseases[J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1116-1121.
share this article
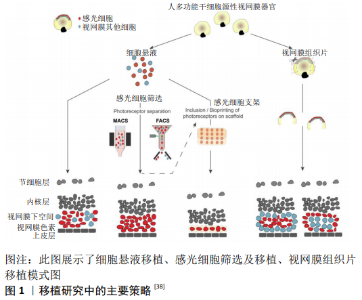
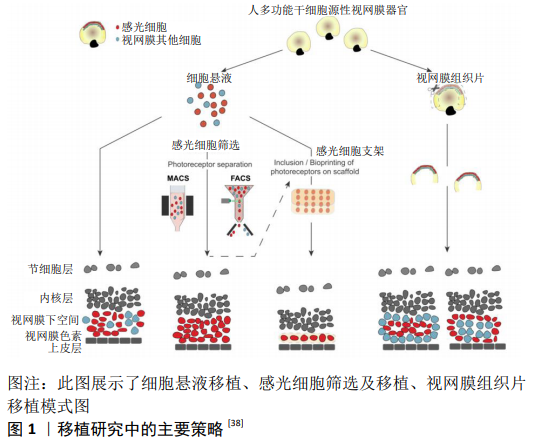
2.1 视网膜变性疾病治疗策略 近年来,致力于视网膜色素变性的新型治疗策略研究大量出现,虽然其中一些已经成功过渡到临床,但对于大多数致盲性疾病仍然没有真正的治疗方法[3],治疗策略包括抗新生血管生长因子的治疗 [10]、基因疗法和基因编辑方法[11]、阻断化学电压钾离子通道[12]、视网膜色素上皮细胞移植、移植生物电子视网膜假体、光发生工具、光敏感开光等。激活内源性干细胞/前体细胞产生新的感光细胞,补充外源性的干细胞/前体细胞在体内分化为感光细胞的研究发展很快[11,13-15],其中外源性细胞作为种子细胞移植到视网膜变性环境中,用于替换晚期视网膜变性中丢失的细胞展现了巨大的优势[16],为视网膜变性疾病提供了新的治疗策略。 2.2 人胚胎干细胞和诱导多能干细胞产生的感光细胞 细胞移植的治疗机制主要为神经营养、免疫调节、细胞替代和突触形成。干细胞具有无限增殖和产生各种细胞的潜能,是细胞替代治疗的一种有前途的细胞,从人胚胎干细胞和诱导多能干细胞中提取视网膜细胞的方法在干细胞培养的基础上进行了探索,并取得了许多成功的结果[17]。细胞移植早期,移植的干细胞/前体细胞主要通过分泌营养因子促进视网膜残留细胞的存活和残余视功能的部分恢复[18],即“bystander”效应;同时移植细胞也可通过免疫调节,抑制小胶质细胞的激活,保护视功能[19]。此外,移植细胞也可释放免疫调节性细胞因子和趋化因子,以及表达免疫调节相关受体以减轻免疫反应[20]。移植干细胞/前体细胞“bystander”的神经营养效应和免疫调节效应在细胞移植早期对视功能的保护起到了关键作用,而细胞移植治疗的长效机制主要基于细胞替代和突触形成,其过程为移植细胞从移植区域迁徙至外核层,分化为感光细胞,与宿主视网膜形成突触连接,并整合进入视网膜环路。种子细胞的选择对整合效果至关重要。多能干细胞因其具有多潜能分化特性及体外无限增殖能力而被广泛认为是种子细胞来源的理想选择。通过胚胎干细胞和诱导性多能干细胞诱导分化形成感光细胞是现今研究的重要方向。 多能干细胞来源的感光细胞在治疗视网膜变性疾病方面展现了巨大的潜力,对于大多数视网膜色素变性患者来说,视杆细胞死亡是疾病早期导致夜盲的主要原因。此外,视杆细胞占感光细胞的90%以上,因此视杆细胞移植是一种适合于视网膜色素变性的治疗方法。到目前为止,胚胎干细胞或诱导性多能干细胞的感光前体细胞移植已经在视网膜变性小鼠模型中完成,且视网膜功能得到一定的改善[21]。 为了克服供体细胞供应的问题,诱导性多能干细胞衍生的感光细胞已被用于临床前移植,多能干细胞诱导分化为感光细胞的诱导方案分为二维培养和三维培养。二维培养方案通常在胚胎干细胞或诱导性多能干细胞贴壁条件下,加入Wnt拮抗剂Dickkopf-1(Dkk-1)、骨形态发生蛋白拮抗剂Noggin、 维甲酸、胰岛素样生长因子1或碱性成纤维生长因子等促进其向感光细胞分化[22]。三维培养方案通常使用多能干细胞无血清悬浮培养形成拟胚体体系,加入特定的细胞分子或小分子[23]。IKEDA等[24]加入体积分数5%血清和activin-A,形成视网膜同源蛋白(Rax)+/配对盒蛋白(Pax6)+视网膜前体细胞的比例约为26%,并继续分化为视锥-视杆同源框转录因子(Crx)+感光祖细胞;LAMBA等[22]在诱导分化3周后约形成80%视网膜前体细胞和12% Crx+感光祖细胞,进一步提高了诱导效率,该诱导方案虽然在相对短的时间内产生高比例的视网膜前体细胞样细胞,然而其使用的基质胶中包含许多不明生长因子。找到可完全明确成分的分化诱导方案对于临床应用至关重要,OSAKADA等[25]首先提出成分确定的诱导方案,对于人胚胎干细胞在早期的神经诱导阶段加入Dkk-1、 Nodal拮抗剂LEFTY-A和Rho激酶抑制剂Y-27632,在感光细胞诱导阶段加入维甲酸和牛磺酸,诱导140-200 d可得到约15% rhodopsin+感光细胞。 EIRAKU等[26]及NAKANO等[27]利用鼠胚胎干细胞及人胚胎干细胞三维培养得到了视杯,开创了多能干细胞三维培养的新时代。EIRAKU等[26]研究显示约80%鼠胚胎干细胞拟胚体含有Rax+细胞,(73.7±2.5)% Rax+聚集体可形成1-4个视泡样结构,其中(57.0±4.7)%可形成视杯样结构,而GONZALEZ-CORDERO等[28]研究表明所有鼠胚胎干细胞形成的拟胚体都至少存在1个神经视网膜样结构,而其中包含有丰富的视杆细胞。NAKANO等[27]对人胚胎干细胞三维诱导的结果显示>70%的人胚胎干细胞拟胚体中含有Rax+细胞,58%-73%的聚集体形成视泡样结构,21%-24%形成视杯样结构;Collin等[29]利用人胚胎干细胞三维培养形成的视杯分离Crx+细胞,在移植后也可以与宿主双极细胞形成突触结构。 多能干细胞在二维环境和三维环境下诱导为视网膜细胞的方案越来越成熟,得到所需细胞的周期越来越短。多能干细胞来源细胞与胚胎发育来源细胞在功能和表型上均具有高度相似性[1,30]。通过三维培养体系得到的感光细胞移植入视网膜变性环境中,可整合入宿主视网膜环路中[28,31],而二维培养来源的感光祖细胞却不能整合入宿主视网膜[32],提示三维培养体系更贴近胚胎发育,从三维培养体系可能得到更优的种子细胞。 2.3 多能干细胞来源的感光细胞选择 2.3.1 通过不同的细胞表面抗原筛选小鼠感光前体细胞 感光前体细胞的选择一直是研究的热点,其中一些研究使用感光细胞特定的启动子,提供腺相关病毒转染细胞来表达绿色荧光蛋白,然而这些通过基因或病毒标记的细胞不能应用于临床。感光前体细胞表面标记物CD73(胞外-5 核苷酸酶)是一种有效的表面抗原,能够特异标记发育中及早期出生的小鼠视网膜感光细胞[33],然后使用流式细胞分选和免疫磁珠分选方法获得大量的感光前体细胞,见图1。LAKOWSKI等[34]发现CD73与另一个细胞表面标记物CD24可同时标记感光前体细胞,CD24主要表达于小鼠胚胎视网膜,在出生后表达迅速下降,研究结果显示移植 CD73阳性细胞相比于CD74/CD24双阳性细胞,与宿主整合效率相同。感光细胞表面CD标记物还有CD133、CD47,其中CD73与CD133主要在Nrl-视杆细胞中高表达,而CD24与CD47主要表达于有丝分裂后期,对于发育中的感光细胞具有特异性[35],作者还发现潜在有害的有丝分裂活性细胞标记物、CD15阴性选择标记物,因此提出了将阳性和阴性方法结合起来作为光感受器前体纯化策略的建议。 2.3.2 通过不同的细胞表面抗原筛选人感光前体细胞 基于小鼠视网膜感光前体细胞标记物的描述,CD24、CD47、CD15并不适用于人类感光前体细胞的选择,但是发现CD73与CD133可表达于人类成人和胎儿的视网膜[36],随后的研究发现CD73是人视锥和视杆细胞的表面标记物,CD73可成功标记视网膜感光细胞,并可将感光细胞从人诱导多能干细胞来源的神经视网膜中成功分离出来,不同的细胞表面标记物被相继发现,如钠依赖性中性氨基酸转运体(SLC6A17)、溶质载体家族40成员1、钾电压门控通道(KCNH2,KCNV2 ),随着RNA测序及单细胞测序技术的发展,不仅有利于发现新的潜在感光细胞标记物,而且有利于了解视网膜的发育阶段,并有助于获得相当于胎儿视网膜细胞的人源性视网膜干细 胞[37]。 2.4 感光细胞替代治疗视网膜变性疾病 研究发现,第11-17天的视网膜片具有良好的分化能力,可分化为含有不同程度内外节的光感受器结构,与细胞悬液移植相似,第18-24天分化的视网膜片表现出结构紊乱,并与单个细胞发生融合[38]。移植整合模式主要与视网膜片结构的完整性有关。事实上,在移植物中存在1个类似于内核层样结构,可以干扰和阻止移植物的感光器与宿主的内核层直接接触,视网膜片中含有不完全内核层,通常这种不完全的内核层围绕在外核层周围,形成玫瑰样结构,能够与宿主双极细胞直接接触,然而这些玫瑰样结构阻断了移植物与宿主视网膜色素上皮的接触[39],见图1。 人胚胎干细胞和诱导性多能干细胞来源的视网膜片移植,在视网膜变性模型中展现出巨大的优势[40],分化第30-65天的视网膜片没有形成成熟的光感受器,而用视网膜类器官制备视网膜片,移植后2-4个月分化为功能性的感光细胞,通过视网膜电生理检测显示视功能改善[41]。最近有研究发现,在免疫缺陷视网膜营养不良模型中,人胚胎干细胞来源的视网膜片移植10个月后,大鼠的视功能依然有改善[42]。 由于视网膜片处在不成熟的发育阶段,移植时视网膜组织富含增殖祖细胞,一般来说在临床环境中具有潜在的危险,但是研究显示未观察到成瘤现象发生[43]。这些研究表明,人胚胎干细胞和诱导性多能干细胞来源的视网膜片移植,在视网膜变性模型中表现出了较好的生长、分化和成熟的能力,同时也指出了使用免疫缺陷模型来检测人多能干细胞来源视网膜片替代治疗的重要性。 2.5 移植策略的挑战与困难 2.5.1 宿主疾病类型对移植效果的影响 移植细胞是否可以整合入宿主视网膜环路与宿主微环境相关。在正常视网膜和变性程度较轻的视网膜微环境中,首先被证实移植细胞可整合入宿主视网膜,随后BARBER等[44]研究表明多种视网膜变性模型(包括严重视网膜变性)也可实现整合。然而,不同疾病类型也对整合效果产生一定影响。视杆祖细胞移植入夜盲模型视杆细胞转导蛋白a敲除小鼠(Gnat1-/-小鼠)的整合细胞数量与移植入正常视网膜环境相似,并且移植细胞可分化形成外节,以及与宿主视网膜形成突触联系。而视杆祖细胞移植入rhodopsin-/-小鼠,整合细胞数目明显减少,且鲜有外节形成。当视杆祖细胞移植入快速视网膜变性模型rd1小鼠后,虽然移植细胞未能分化形成成熟感光细胞的形态,但是整合细胞数量多,且可促使宿主视功能恢复[44-46]。 2.5.2 外界膜完整性对于移植效果的影响 对整合极其重要的是供体细胞从移植区域(通常是视网膜下腔)通过外界膜迁徙至外核层[47]。现认为这一过程中的两大决定性因素为外界膜的完整性和宿主视网膜胶质化的程度[44,48-50]。Müller胶质细胞的终端在感光细胞内节之间形成的黏着连接构成了外界膜。外界膜是阻碍移植细胞来源感光细胞整合入宿主视网膜的一大屏障。因此破坏外界膜的完整性有助于提高整合效率,其方法包括药物干预(如胶质毒素DL-α-氨基己二酸)和基因沉默外界膜相关蛋白ZO-1[44,49-50],然而这两类策略都无法直接用于临床治疗。破坏外界膜的药物毒性也可直接损害Müller胶质细胞,并且外界膜和视网膜色素上皮层都表达黏着连接蛋白ZO-1,沉默ZO-1可破坏外界膜的同时也会引起视网膜色素上皮细胞的变性,故ZO-1也不是理想的特异性靶点。 2.5.3 反应性胶质增生对移植细胞的影响 反应性胶质增生是影响中枢神经系统修复和再生的主要因素;另一方面,反应性胶质增生也是促进剩余神经元存活的保护因素,并可引起神经元重塑[51]。在视网膜中,反应性胶质增生主要来源于Müller胶质细胞。Müller胶质细胞增生会引起vimentin和胶质纤维酸性蛋白上调,Müller胶质细胞胞体肥大,以及抑制性细胞外基质分子的堆积,如硫酸软骨素蛋白聚糖[52]。在变性环境下,Müller胶质细胞反应性胶质增生所产生的胶质瘢痕以及抑制性细胞外基质分子都会阻碍移植细胞整合入宿主视网膜中。据此,降低宿主视网膜胶质化程度可改善移植细胞的整合效果。GFAP-/-Vim-/-小鼠不表达胶质相关蛋白GFAP和vimentin,其胶质瘢痕的形成显著减少,在这样的环境中移植细胞的迁徙明显增加[48,53]。通过降低硫酸软骨素蛋白聚糖的浓度也可促进整合效果的提高。硫酸软骨素蛋白聚糖结合了许多细胞外基质蛋白和生长因子,对调节细胞黏附和迁徙起到了重要作用。在中枢神经系统受到损伤后,硫酸软骨素蛋白聚糖出现上调,并抑制神经元轴突的再生,而视网膜损伤后硫酸软骨素蛋白聚糖的变化趋势尚不明确,在不同的变性环境中硫酸软骨素蛋白聚糖家族中的不同成分呈现了不同的表达方式,如在两种视网膜营养不良大鼠模型中聚集蛋白聚糖呈现上调[54],而在rd1小鼠和rhodopsin-/-小鼠中神经糖蛋白呈现上调[55]。采用可分解多种硫酸软骨素蛋白聚糖的广谱软骨素酶ChABC,可有效促进受损脊髓的轴突再生。ChABC的应用也可水解视网膜变性环境中堆积的硫酸软骨素蛋白聚糖,促进整合效果的提高[44]。基质金属蛋白酶可降解细胞外基质的多种成分,包括胶原、纤连蛋白、层粘连蛋白和多种蛋白聚糖等。研究表明,应用基质金属蛋白酶2也可促进移植细胞迁徙入宿主视网膜[56]。rd1小鼠既可出现严重的胶质瘢痕形成,也会出现外界膜完整性下降[44],但是胶质增生不仅仅对视功能起到不利影响,一定程度的胶质增生也会促进移植细胞和宿主残留视锥细胞的存活[57]。 2.5.4 免疫反应对移植效果的影响 虽然视网膜是免疫赦免器官,对外来物质(移植细胞)较少产生免疫反应,但是在视网膜变性情况下,除了移植相关损伤外,免疫反应可能更加明显,许多视网膜退行性疾病,如年龄相关性黄斑变性及遗传性视网膜变性,都会导致视网膜色素上皮细胞的丢失,这不仅会干扰这些细胞分泌免疫抑制因子,还可能导致血-视网膜屏障破坏,进一步削弱视网膜的免疫赦免;此外,年龄相关性黄斑变性疾病在发展过程中免疫调节反应加强,可进一步加剧免疫排斥反应。细胞移植注射到视网膜下腔后,会引起视网膜下空间炎性细胞浸润,如视网膜小胶质细胞和抗原提呈细胞浸润,T细胞、B细胞激活并产生免疫反应[58]。 免疫抑制可能有助于提高临床细胞移植的效率。WEST等[59]发现巨噬细胞及T细胞可减少感光细胞的整合及突出形成,而给予淋巴细胞活性抑制剂和环孢素A等免疫抑制可显著增加感光细胞的存活率,在免疫缺陷小鼠中,特别是在白细胞介素2受体敲除的小鼠中发现多能干细胞来源的感光细胞移植成功率明显提高,可成功分化为成熟的感光细胞并改善视功能[60]。"
| [1] JIN ZB, GAO ML, DENG WL, et al. Stemming retinal regeneration with pluripotent stem cells. Prog Retin Eye Res. 2019;69:38-56. [2] GASPARINI SJ, LLONCH S, BORSCH O, et al. Transplantation of photoreceptors into the degenerative retina: Current state and future perspectives. Prog Retin Eye Res. 2019;69:1-37. [3] ROSKA B, SAHEL JA. Restoring vision. Nature. 2018;557(7705):359-367. [4] SCHOLL HP, STRAUSS RW, SINGH MS, et al. Emerging therapies for inherited retinal degeneration. Sci Transl Med. 2016;8(368):368rv6. [5] PASCOLINI D, MARIOTTI SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012;96(5):614-618. [6] GEHRS KM, ANDERSON DH, JOHNSON LV, et al. Age-related macular degeneration--emerging pathogenetic and therapeutic concepts. Ann Med. 2006;38(7):450-471. [7] DAMIANI D, NOVELLI E, MAZZONI F, et al. Undersized dendritic arborizations in retinal ganglion cells of the rd1 mutant mouse: a paradigm of early onset photoreceptor degeneration. J Comp Neurol. 2012;520(7):1406-1423. [8] MAZZONI F, NOVELLI E, STRETTOI E. Retinal ganglion cells survive and maintain normal dendritic morphology in a mouse model of inherited photoreceptor degeneration. J Neurosci. 2008;28(52):14282-14292. [9] SANTOS A, HUMAYUN MS, DE JUAN E JR, et al. Preservation of the inner retina in retinitis pigmentosa. A morphometric analysis. Arch Ophthalmol. 1997;115(4):511-515. [10] EHLKEN C, JUNGMANN S, BÖHRINGER D, et al. Switch of anti-VEGF agents is an option for nonresponders in the treatment of AMD. Eye (Lond). 2014;28(5):538-545. [11] BURNIGHT ER, GIACALONE JC, COOKE JA, et al. CRISPR-Cas9 genome engineering: Treating inherited retinal degeneration. Prog Retin Eye Res. 2018;65:28-49. [12] VAN GELDER RN. Photochemical approaches to vision restoration. Vision Res. 2015;111(Pt B):134-141. [13] WILKEN MS, REH TA. Retinal regeneration in birds and mice. Curr Opin Genet Dev. 2016;40:57-64. [14] YAO K, QIU S, WANG YV, et al. Restoration of vision after de novo genesis of rod photoreceptors in mammalian retinas. Nature. 2018; 560(7719):484-488. [15] JORSTAD NL, WILKEN MS, GRIMES WN, et al. Stimulation of functional neuronal regeneration from Müller glia in adult mice. Nature. 2017; 548(7665):103-107. [16] DA CRUZ L, FYNES K, GEORGIADIS O, et al. Phase 1 clinical study of an embryonic stem cell-derived retinal pigment epithelium patch in age-related macular degeneration. Nat Biotechnol. 2018;36(4):328-337. [17] JIN ZB, TAKAHASHI M. Generation of retinal cells from pluripotent stem cells. Prog Brain Res. 2012;201:171-181. [18] MA Y, HAN X, DE CASTRO RB, et al. Analysis of the bystander effect in cone photoreceptors via a guided neural network platform. Sci Adv. 2018;4(5):eaas9274. [19] LI Z, ZENG Y, CHEN X, et al. Neural stem cells transplanted to the subretinal space of rd1 mice delay retinal degeneration by suppressing microglia activation. Cytotherapy. 2016;18(6):771-784. [20] LUI KO, BOYD AS, COBBOLD SP, et al. A role for regulatory T cells in acceptance of ESC-derived tissues transplanted across an major histocompatibility complex barrier. Stem Cells. 2010;28(10):1905-1914. [21] PEARSON RA, BARBER AC, RIZZI M, et al. Restoration of vision after transplantation of photoreceptors. Nature. 2012;485(7396):99-103. [22] LAMBA DA, KARL MO, WARE CB, et al. Efficient generation of retinal progenitor cells from human embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103(34):12769-12774. [23] LAMBA DA, REH TA. Microarray characterization of human embryonic stem cell--derived retinal cultures. Invest Ophthalmol Vis Sci. 2011; 52(7):4897-4906. [24] IKEDA H, OSAKADA F, WATANABE K, et al. Generation of Rx+/Pax6+ neural retinal precursors from embryonic stem cells. Proc Natl Acad Sci U S A. 2005;102(32):11331-11336. [25] OSAKADA F, IKEDA H, MANDAI M, et al. Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nat Biotechnol. 2008;26(2):215-224. [26] EIRAKU M, TAKATA N, ISHIBASHI H, et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 2011;472(7341): 51-56. [27] NAKANO T, ANDO S, TAKATA N, et al. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell. 2012; 10(6):771-785. [28] GONZALEZ-CORDERO A, WEST EL, Pearson RA, et al. Photoreceptor precursors derived from three-dimensional embryonic stem cell cultures integrate and mature within adult degenerate retina. Nat Biotechnol. 2013;31(8):741-747. [29] COLLIN J, ZERTI D, QUEEN R, et al. CRX Expression in Pluripotent Stem Cell-Derived Photoreceptors Marks a Transplantable Subpopulation of Early Cones. Stem Cells. 2019;37(5):609-622. [30] SINGH R, CUZZANI O, BINETTE F, et al. Pluripotent Stem Cells for Retinal Tissue Engineering: Current Status and Future Prospects. Stem Cell Rev Rep. 2018;14(4):463-483. [31] ZOU T, GAO L, ZENG Y, et al. Organoid-derived C-Kit+/SSEA4- human retinal progenitor cells promote a protective retinal microenvironment during transplantation in rodents. Nat Commun. 2019;10(1):1205. [32] WEST EL, GONZALEZ-CORDERO A, HIPPERT C, et al. Defining the integration capacity of embryonic stem cell-derived photoreceptor precursors. Stem Cells. 2012;30(7):1424-1435. [33] EBERLE D, SCHUBERT S, POSTEL K, et al. Increased integration of transplanted CD73-positive photoreceptor precursors into adult mouse retina. Invest Ophthalmol Vis Sci. 2011;52(9):6462-6471. [34] LAKOWSKI J, HAN YT, PEARSON RA, et al. Effective transplantation of photoreceptor precursor cells selected via cell surface antigen expression. Stem Cells. 2011;29(9):1391-1404. [35] LAKOWSKI J, GONZALEZ-CORDERO A, WEST EL, et al. Transplantation of Photoreceptor Precursors Isolated via a Cell Surface Biomarker Panel From Embryonic Stem Cell-Derived Self-Forming Retina. Stem Cells. 2015;33(8):2469-2482. [36] LAKOWSKI J, WELBY E, BUDINGER D, et al. Isolation of Human Photoreceptor Precursors via a Cell Surface Marker Panel from Stem Cell-Derived Retinal Organoids and Fetal Retinae. Stem Cells. 2018; 36(5):709-722. [37] POSTEL K, BELLMANN J, SPLITH V, et al. Analysis of cell surface markers specific for transplantable rod photoreceptors. Mol Vis. 2013;19: 2058-2067. [38] GAGLIARDI G, BEN M’BAREK K, GOUREAU O. Photoreceptor cell replacement in macular degeneration and retinitis pigmentosa: A pluripotent stem cell-based approach. Prog Retin Eye Res. 2019;71: 1-25. [39] ASSAWACHANANONT J, MANDAI M, OKAMOTO S, et al. Transplantation of embryonic and induced pluripotent stem cell-derived 3D retinal sheets into retinal degenerative mice. Stem Cell Reports. 2014; 2(5): 662-674. [40] TU HY, WATANABE T, SHIRAI H, et al. Medium- to long-term survival and functional examination of human iPSC-derived retinas in rat and primate models of retinal degeneration. EBioMedicine. 2019;39: 562-574. [41] ZHONG X, GUTIERREZ C, XUE T, et al. Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nat Commun. 2014;5:4047. [42] MCLELLAND BT, LIN B, MATHUR A, et al. Transplanted hESC-Derived Retina Organoid Sheets Differentiate, Integrate, and Improve Visual Function in Retinal Degenerate Rats. Invest Ophthalmol Vis Sci. 2018; 59(6):2586-2603. [43] SHIRAI H, MANDAI M, MATSUSHITA K, et al. Transplantation of human embryonic stem cell-derived retinal tissue in two primate models of retinal degeneration. Proc Natl Acad Sci U S A. 2016;113(1):E81-90. [44] BARBER AC, HIPPERT C, DURAN Y, et al. Repair of the degenerate retina by photoreceptor transplantation. Proc Natl Acad Sci U S A. 2013; 110(1):354-359. [45] LI T, LEWALLEN M, CHEN S, et al. Multipotent stem cells isolated from the adult mouse retina are capable of producing functional photoreceptor cells. Cell Res. 2013;23(6):788-802. [46] SINGH MS, CHARBEL ISSA P, BUTLER R, et al. Reversal of end-stage retinal degeneration and restoration of visual function by photoreceptor transplantation. Proc Natl Acad Sci U S A. 2013;110(3):1101-1106. [47] WARRE-CORNISH K, BARBER AC, SOWDEN JC, et al. Migration, integration and maturation of photoreceptor precursors following transplantation in the mouse retina. Stem Cells Dev. 2014;23(9): 941-954. [48] KINOUCHI R, TAKEDA M, YANG L, et al. Robust neural integration from retinal transplants in mice deficient in GFAP and vimentin. Nat Neurosci. 2003;6(8):863-868. [49] PEARSON RA, BARBER AC, WEST EL, et al. Targeted disruption of outer limiting membrane junctional proteins (Crb1 and ZO-1) increases integration of transplanted photoreceptor precursors into the adult wild-type and degenerating retina. Cell Transplant. 2010;19(4): 487-503. [50] WEST EL, PEARSON RA, TSCHERNUTTER M, et al. Pharmacological disruption of the outer limiting membrane leads to increased retinal integration of transplanted photoreceptor precursors. Exp Eye Res. 2008;86(4):601-611. [51] KARL MO, REH TA. Regenerative medicine for retinal diseases: activating endogenous repair mechanisms. Trends Mol Med. 2010;16(4):193-202. [52] KARL MO. The potential of stem cell research for the treatment of neuronal damage in glaucoma. Cell Tissue Res. 2013;353(2):311-325. [53] VERARDO MR, LEWIS GP, TAKEDA M, et al. Abnormal reactivity of muller cells after retinal detachment in mice deficient in GFAP and vimentin. Invest Ophthalmol Vis Sci. 2008;49(8):3659-3665. [54] CHEN LF, FITZGIBBON T, HE JR, et al. Localization and developmental expression patterns of CSPG-cs56 (aggrecan) in normal and dystrophic retinas in two rat strains. Exp Neurol. 2012;234(2):488-498. [55] ROESCH K, STADLER MB, CEPKO CL. Gene expression changes within Müller glial cells in retinitis pigmentosa. Mol Vis. 2012;18:1197-1214. [56] YAO J, TUCKER BA, ZHANG X, et al. Robust cell integration from co-transplantation of biodegradable MMP2-PLGA microspheres with retinal progenitor cells. Biomaterials. 2011;32(4):1041-1050. [57] LEE ES, YU SH, JANG YJ, et al. Transplantation of bone marrow-derived mesenchymal stem cells into the developing mouse eye. Acta Histochem Cytochem. 2011;44(5):213-221. [58] SUGITA S, MAKABE K, FUJII S, et al. Detection of Retinal Pigment Epithelium-Specific Antibody in iPSC-Derived Retinal Pigment Epithelium Transplantation Models. Stem Cell Reports. 2017;9(5): 1501-1515. [59] WEST EL, PEARSON RA, BARKER SE, et al. Long-term survival of photoreceptors transplanted into the adult murine neural retina requires immune modulation. Stem Cells. 2010;28(11):1997-2007. [60] ZHU J, CIFUENTES H, REYNOLDS J, et al. Immunosuppression via Loss of IL2rγ Enhances Long-Term Functional Integration of hESC-Derived Photoreceptors in the Mouse Retina. Cell Stem Cell. 2017;20(3): 374-384.e5. [61] MACLAREN RE, PEARSON RA, MACNEIL A, et al. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444(7116): 203-207. [62] BARTSCH U, ORIYAKHEL W, KENNA PF, et al. Retinal cells integrate into the outer nuclear layer and differentiate into mature photoreceptors after subretinal transplantation into adult mice. Exp Eye Res. 2008; 86(4):691-700. |
| [1] | Lin Qingfan, Xie Yixin, Chen Wanqing, Ye Zhenzhong, Chen Youfang. Human placenta-derived mesenchymal stem cell conditioned medium can upregulate BeWo cell viability and zonula occludens expression under hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 4970-4975. |
| [2] | Pu Rui, Chen Ziyang, Yuan Lingyan. Characteristics and effects of exosomes from different cell sources in cardioprotection [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 1-. |
| [3] | Zhang Chao, Lü Xin. Heterotopic ossification after acetabular fracture fixation: risk factors, prevention and treatment progress [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1434-1439. |
| [4] | Zhou Jihui, Li Xinzhi, Zhou You, Huang Wei, Chen Wenyao. Multiple problems in the selection of implants for patellar fracture [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1440-1445. |
| [5] | Wang Debin, Bi Zhenggang. Related problems in anatomy mechanics, injury characteristics, fixed repair and three-dimensional technology application for olecranon fracture-dislocations [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1446-1451. |
| [6] | Ji Zhixiang, Lan Changgong. Polymorphism of urate transporter in gout and its correlation with gout treatment [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1290-1298. |
| [7] | Yuan Mei, Zhang Xinxin, Guo Yisha, Bi Xia. Diagnostic potential of circulating microRNA in vascular cognitive impairment [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1299-1304. |
| [8] | Zhang Xiumei, Zhai Yunkai, Zhao Jie, Zhao Meng. Research hotspots of organoid models in recent 10 years: a search in domestic and foreign databases [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1249-1255. |
| [9] | Wang Zhengdong, Huang Na, Chen Jingxian, Zheng Zuobing, Hu Xinyu, Li Mei, Su Xiao, Su Xuesen, Yan Nan. Inhibitory effects of sodium butyrate on microglial activation and expression of inflammatory factors induced by fluorosis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1075-1080. |
| [10] | Wang Xianyao, Guan Yalin, Liu Zhongshan. Strategies for improving the therapeutic efficacy of mesenchymal stem cells in the treatment of nonhealing wounds [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1081-1087. |
| [11] | Wan Ran, Shi Xu, Liu Jingsong, Wang Yansong. Research progress in the treatment of spinal cord injury with mesenchymal stem cell secretome [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1088-1095. |
| [12] | Liao Chengcheng, An Jiaxing, Tan Zhangxue, Wang Qian, Liu Jianguo. Therapeutic target and application prospects of oral squamous cell carcinoma stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1096-1103. |
| [13] | Zhao Min, Feng Liuxiang, Chen Yao, Gu Xia, Wang Pingyi, Li Yimei, Li Wenhua. Exosomes as a disease marker under hypoxic conditions [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1104-1108. |
| [14] | Xie Wenjia, Xia Tianjiao, Zhou Qingyun, Liu Yujia, Gu Xiaoping. Role of microglia-mediated neuronal injury in neurodegenerative diseases [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1109-1115. |
| [15] | Jiao Hui, Zhang Yining, Song Yuqing, Lin Yu, Wang Xiuli. Advances in research and application of breast cancer organoids [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1122-1128. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||