Chinese Journal of Tissue Engineering Research ›› 2021, Vol. 25 ›› Issue (7): 1104-1108.doi: 10.3969/j.issn.2095-4344.2177
Previous Articles Next Articles
Exosomes as a disease marker under hypoxic conditions
Zhao Min1, Feng Liuxiang2, Chen Yao1, Gu Xia1, Wang Pingyi1, Li Yimei1, Li Wenhua1
- 1Xizang Minzu University, Xianyang 712082, Shaanxi Province, China; 2People’s Hospital of Yulong Naxi Autonomous County of Lijiang City, Yulong Naxi Autonomous County 674100, Yunnan Province, China
-
Received:2020-03-12Revised:2020-03-18Accepted:2020-05-09Online:2021-03-08Published:2020-12-09 -
Contact:Li Wenhua, Professor, Master’s supervisor, Xizang Minzu University, Xianyang 712082, Shaanxi Province, China -
About author:Zhao Min, Master candidate, Xizang Minzu University, Xianyang 712082, Shaanxi Province, China Feng Liuxiang, Physician, People’s Hospital of Yulong Naxi Autonomous County of Lijiang City, Yulong Naxi Autonomous County 674100, Yunnan Province, China -
Supported by:the National Natural Science Foundation of China in 2017, No. 81760332; the Scientific Research Program of Shaanxi Provincial Department of Education in 2020; Head of the Joint Project of the Department of Science and Technology of Tibet in 2020 (Research on the Role and Mechanism of Hongjingtian, Tibetan Medicine, Anti-COVID-19 “Inflammation Storm”)
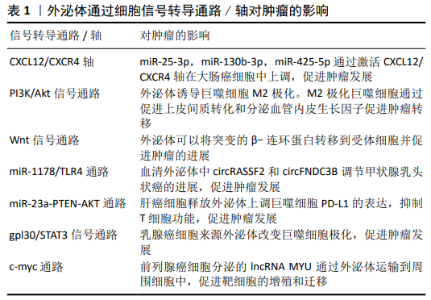
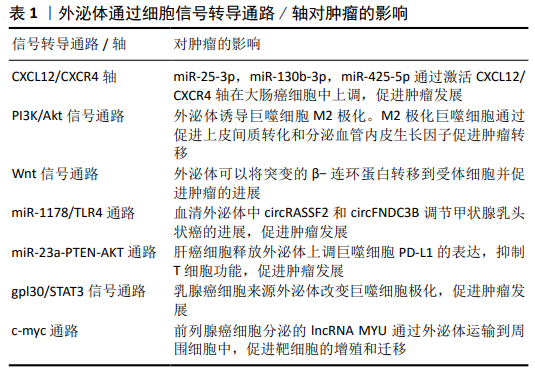
2.1 外泌体与低氧诱导因子1α 低氧可以促进细胞中低氧诱导因子1α和低氧诱导因子2α的表达和核转位,导致葡萄糖转运蛋白和表皮生长因子受体蛋白水平增加,继而受体表达的改变(减少或增加)可以促进质膜重塑,受体表达增加可以直接促进受体激活和内化,从而诱导内吞作用,最终促进外泌体释放。低氧刺激的外泌体释放依赖于低氧诱导因子1α。然而,低氧诱导因子1α对外泌体功能的调节是一个新的研究领域,低氧诱导因子1α作用于外泌体的直接机制以及低氧诱导因子1α调节外泌体形成、内容物选择、转运和释放的作用尚未确定。多种细胞衍生的外泌体通过减少氧化应激、抗纤维化、促进血管生成、抑制细胞凋亡等在不同类型的低氧疾病中起保护和治疗作用。LV等[10]研究发现在低氧条件下低氧诱导因子1α过表达的间充质干细胞分泌的外泌体增强了血管生成和血管通透性。LI等[11]将肾小管上皮细胞暴露于缺氧条件下,以促进miRNA-23a外泌体的释放,然后将其注射到未受损伤的肾实质,导致体内炎症浸润增加。在大鼠肾近端肾小管细胞缺氧时,能显著促进外泌体的产生和分泌,缺氧细胞来源外泌体对肾小管细胞损伤具有保护作用[12]。研究发现低氧诱导因子1可以诱导低氧处理的肾小管上皮细胞产生外泌体,并能调节外泌体的含量以改变受体细胞的关键功能[13-14]。此外,WANG等[5]报道低氧诱导因子可以通过增加缺氧环境下Rab22a的活性来调节外泌体的形成、运输和膜融合。CHEN等[15]发现在缺氧条件下卵巢癌细胞来源的外泌体通过miRNAs诱导M2巨噬细胞极化,从而促进卵巢癌细胞的增殖和迁移,低氧诱导因子在这一过程中起着重要作用。ZHANG等[16]在大鼠股骨骨折模型中发现脐带间充质干细胞来源外泌体促进低氧诱导因子1α和血管内皮生长因子水平增加,从而增强血管生成。以上反映了外泌体与低氧诱导因子存在着直接或间接的关系。 2.2 外泌体是一种介导低氧细胞间信号传递的新载体 多种疾病如肿瘤、心血管疾病、感染与炎症等都处于低氧的细胞内环境,并且外泌体有多种方式参与细胞间的细息传递。外泌体中含有蛋白质、脂类和核酸(如功能性的mRNAs),可作为细胞间信号传递的新载体[17-21]。下面将从上述这几个常见病来阐述外泌体在低氧细胞间的信息传递,以期为高原低氧环境下开展研究外泌体提供新依据。 2.2.1 外泌体在肿瘤细胞间的信息通讯 外泌体可介导肿瘤细胞微环境之间相互通信。HAN等[22]在肝细胞癌研究中发现Vps4A影响外泌体的释放,其通过与CHMP4B和β-catenin的相互作用,调节β-catenin的PM定位和外泌体排序,降低β-catenin信号转导,从而抑制肝癌的上皮-间质转化和转移。CHEN等[23]研究发现肝细胞癌小鼠血浆外泌体和组织中circ-0051443表达明显低于健康对照组。Circ-0051443通过外泌体从正常细胞向肝癌细胞传递,通过促进细胞凋亡和抑制细胞周期进程来实现抑制癌细胞生长。外泌体circ-0051443通过上调BAK1表达降低裸鼠移植瘤的质量和体积,外泌体circ-0051443可以作为肝癌的预测因子和潜在治疗靶点。胰腺癌是一种致命的恶性肿瘤,最近研究发现骨髓间充质干细胞来源外泌体(miR-126-3p)通过下调ADAM9表达抑制胰腺癌的发生[24]。YUE等[25]研究发现缺氧性胶质母细胞瘤细胞特异性分泌的外泌体miR-301a通过TCEAL7(胶质母细胞瘤中的一种抑癌基因)激活Wnt/β-catenin信号通路,从而可能抑制癌症发展。 外泌体还可以促进肿瘤的发展,肿瘤相关巨噬细胞(TAMs)是与肿瘤转移相关的重要免疫细胞。在大肠癌中外泌体(miR-25-3p,miR-130b-3p,miR-425-5p)通过激活PI3K/Akt信号通路调节PTEN表达,诱导巨噬细胞M2极化,通过促进上皮间质转化和分泌血管内皮生长因子促进肿瘤转移[26]。此外,KALRA等[27]在大肠癌研究中首次发现外泌体可以将突变的β-连环蛋白转移到受体细胞并促进肿瘤的进展,这一过程是通过激活Wnt信号通路来实现的。CHEN等[28]研究发现乳腺癌外泌体lncRNAs可作为信号转导元件,在免疫细胞和肿瘤细胞之间传递,以促进癌症有氧糖酵解。最新研究发现,甲状腺乳头状癌的血清外泌体circRASSF2和circFNDC3B 通过miR-1178/TLR4途径调节甲状腺乳头状癌的进展,且呈正相关[29-30]。内质网应激的肝癌细胞释放外泌体可以上调巨噬细胞PD-L1的表达,进而通过miR-23a-PTEN-AKT途径抑制T细胞功能,促进肿瘤发展[31]。HAM等[32]研究发现乳腺癌细胞来源外泌体可以通过gpl30/STAT3信号通路改变巨噬细胞极化。WANG等[33]研究发现lncRNA MYU至少部分通过miR-184/c-Myc轴在前列腺癌中起癌基因的作用,并可能作为潜在的诊断生物标记物和治疗靶点。见表1。 2.2.2 外泌体在心脑血管疾病间细胞通讯 GAO等[34]用差速离心法从心肌梗死小鼠血清中分离外泌体,其通过激活ERK1/2促进脂肪间充质干细胞增殖。功能获得和丧失研究证实miR-1956通过下调Notch-1刺激脂肪间充质干细胞介导的血管生成和旁分泌血管内皮生长因子信号传导,从而起到修复心肌作用。研究发现心肌缺血患者的冠状动脉血清外泌体中的miR-939 介导NO信号通路促使血管新生[35]。自发性高血压大鼠血管外膜成纤维细胞来源外泌体向血管平滑肌细胞转移血管紧张素转换酶,增加血管平滑肌细胞AngⅡ水平,激活AngⅡ受体,从而促进血管平滑肌细胞迁移[36]。最近XU等[37]研究发现褪黑素处理的血管平滑肌细胞来源外泌体可以通过miR-204/miR-211以旁分泌的方式减弱血管钙化和老化,对高血压、动脉粥样硬化的血管有保护作用。 2.2.3 外泌体在炎症时细胞间通讯 研究发现,在炎症与感染的时候,也会出现类似的信号交流。气道重塑在气管炎症中尤其重要,GUPTA等[38]研究发现外泌体可以在气道上皮进行细胞间信息传递,有助于气道上皮细胞重塑。急性肺损伤相关的肺部炎症与巨噬细胞过度活化有关。急性肺损伤小鼠血清外泌体将miR-155传递给巨噬细胞,刺激核因子κB活化,并诱导肿瘤坏死因子α和白细胞介素的产生[39]。此外,血清外泌体来源的miR-155分别通过靶向SHIP1和SOCS1促进巨噬细胞增殖和炎症反应,从而促进炎症发生。刘超[40]利用豚鼠哮喘模型研究血清外泌体在其中发挥的作用,发现血清外泌体可能通过提高膜蛋白HSP70表达,且活化TLR4/NF-kB信号通路来发挥促炎反应,影响哮喘的发展。在类风湿关节炎中,WANG等[41]研究发现血清来源外泌体 miR-548a-3p通过调节TLR4/NF-κB信号通路,抑制pTHP-1细胞的增殖和活化,从而有益于抑制炎症。 2.3 外泌体作为疾病的生物标志物 外泌体不仅在肿瘤、心血管疾病、感染与炎症等常见病多发病中作为重要的信息传递分子,而且在疾病的发生发展中尤为重要,且表现出特异性,可以作为新的疾病生物标志物。 2.3.1 外泌体作为肿瘤标志物 目前肿瘤是人类需要克服的一大难题,致残率和死亡率均居前列,但是没有一种有效的诊断和预后措施。肿瘤所处的环境主要就是缺氧,因此在这种环境下外泌体对肿瘤的影响必不可少,可作为肿瘤生物标志物,为临床早期诊断、治疗和预后提供新的临床数据。 外泌体中含有多种核酸,其中肿瘤外泌体中非编码RNA可作为生物标志物。研究发现涎腺腺样囊性癌(SACC)患者血浆中分离的外泌体中MRPL23-AS1水平较高,且富含MRPL23-AS1的外泌体增加了微血管的通透性,并促进了体内涎腺腺样囊性癌的转移[42],揭示了MRPL23-AS1可能为生物标记物。研究发现,肝细胞癌血清外泌体中的LncRNA ZFAS1明显高表达[43],可作为生物标志物。最近研究发现,lncRNA PCAT1存在于食管鳞状细胞癌的外泌体中,通过激活miR-326(一种肿瘤抑制因子)促进食管鳞状细胞癌细胞增殖,可能作为一个非侵入性生物标记物[44]。HU等[45]研究发现结直肠癌中的6种外泌体lncRNA (LNCV6_116109、LNCV6_98390、LNCV6_38772、LNCV6_108266、LNCV6_84003、LNCV6_98602)在结直肠癌患者血浆中的表达明显上调,可作为结直肠癌早期诊断的潜在非侵袭性生物标志物。张好良等[46]研究胃癌时发现血清外泌体LncRNA PN2-4低表达,并且表达越低肿瘤恶性程度越高,预后越差,可作为肿瘤生物标记物。 肿瘤外泌体中miRNA也可作为生物标记物。SU等[47]研究卵巢癌时发现血清外泌体中的miR-1307和miR-375高表达,其中miR-1307与肿瘤的恶化程度有关,表达越高恶化程度越高。miR-375与肿瘤的淋巴转移有关,表达越高淋巴转移越强。因此可作为肿瘤的生物标记物。ZHENG等[48]对宫颈上皮内瘤样病变和宫颈癌患者血浆标本进行miRNA测序,发现血浆外泌体miR-30d-5p和let-7d-3p是宫颈癌及其前体无创筛查的有价值的诊断生物标志物,但是临床诊断需要大样本的进一步验证。研究发现血清外泌体miR-20b-5p可能参与非小细胞肺癌的发病机制,具有较高的临床诊断价值,可以作为辅助非小细胞肺癌诊断的新型生物标志物[49]。结肠癌的早期诊断对提高患者的生存率和生活质量具有重要的临床意义。研究发现,在结肠癌患者中具有明显的外泌体miRNA谱,可作为早期检测结肠癌的一个有前途的生物标记物,但还需要大量数据证实[50]。SHAO等[51]对胶质瘤进行研究发现外泌体miR-454-3p的高表达或低表达与胶质瘤预后有关,且有特异性,可作为生物标志物,是潜在的治疗靶点。 2.3.2 外泌体作为心脑血管疾病生物标志物 众所周知,心脑血管疾病与低氧密不可分,低氧会诱导心脑血管疾病,反过来心脑血管疾病会加重缺氧。因此找到一个及时诊断心脑血管疾病的标记物非常重要。张海涛等[52]研究发现稳定性心绞痛患者血清外泌体中的miR-5195-3p、miR-328和miR-130a-3p 较正常人显著上调,而急性ST段抬高型心肌梗死患者血清外泌体中 miR-4787-3p、miR-423-5p和miR-151b较正常人显著上调,与冠心病的发展有着重要关系,可作为生物标志物。以往研究发现,急性心肌梗死患者血清外泌体miR-146b-5p的含量比正常人明显高表达,对诊断急性心肌梗死有重要意义[53]。人体衰老时,心脏会发生纤维化,导致缺氧加重。YANG等[54]利用心肌纤维化模型研究发现,随着年龄的增大,血清外泌体表面的HSP70表达量减少,心肌纤维化加重,不仅可作为诊断的生物标记物,还给心肌纤维化的治疗提供了新的思路。 2.3.3 外泌体作为炎症生物标志物 当机体发生炎症后局部会发生缺氧,导致炎症更加严重。血清外泌体在机体发生炎症后有所变化,作为生物标志物在临床上有着重要的作用。 在研究丙型病毒性肝炎时,发现其血清外泌体miR-122显著降低且与谷草转氨酶和谷丙转氨酶表达水平呈负相关,但是目前只可能作为肝损伤的生物标记物,还不能成为丙型肝炎的治疗效果监测标志物[55]。研究发现,炎症相关mRNA谱分析显示IgA肾病患者外泌体趋化因子(C-C基序)配体2(CCL2)表达上调,且CCL2在IgA肾病患者中高度表达,外泌体CCL2 mRNA是反映IgA肾病活动性肾组织损伤和肾功能恶化的重要生物标志物[56]。LIAO等[57]对丙型肝炎病毒感染的类风湿性关节炎患者进行研究发现,外泌体miR-155水平与丙型肝炎病毒载量呈负相关且表达量较高,miR-155可能成为潜在的诊断生物标志物或治疗靶点。随后又发现,类风湿性关节炎患者血清外泌体miR-548a-3p明显低于健康人,可能是预测类风湿性关节炎的生物标记物[41]。"
| [1] JOHNSTONE RM, ADAM M, HAMMOND JR, et al. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262(19):9412-9420. [2] PIRONTI G, STRACHAN RT, ABRAHAM D,et al. Circulating Exosomes Induced by Cardiac Pressure Overload Contain Functional Angiotensin II Type 1 Receptors. Circulation. 2015;131(24):2120-2130. [3] YANG J, WEI F, SCHAFER C, et al. Detection of tumor cell-specific mRNA and protein in exosome-like microvesicles from blood and saliva. PLoS One. 2014;9(11):e110641. [4] HO DH, YI S, SEO H, et al. Increased DJ-1 in urine exosome of Korean males with Parkinson’s disease. Biomed Res Int. 2014;2014:704678. [5] WANG T, GILKES DM, TAKANO N, et al. Hypoxia-inducible factors and RAB22A mediate formation of microvesicles that stimulate breast cancer invasion and metastasis. Proc Natl Acad Sci U S A. 2014;111(31): E3234-3242. [6] ZHAO L, LUO H, LI X, et al. Exosomes Derived from Human Pulmonary Artery Endothelial Cells Shift the Balance between Proliferation and Apoptosis of Smooth Muscle Cells. Cardiology. 2017;137(1):43-53. [7] SANO S, IZUMI Y, YAMAGUCHI T, et al. Lipid synthesis is promoted by hypoxic adipocyte-derived exosomes in 3T3-L1 cells. Biochem Biophys Res Commun. 2014;445(2):327-333. [8] BORGES FT, MELO SA, ÖZDEMIR BC, et al. TGF-β1-containing exosomes from injured epithelial cells activate fibroblasts to initiate tissue regenerative responses and fibrosis. J Am Soc Nephrol. 2013;24(3):385-392. [9] AGARWAL U, GEORGE A, BHUTANI S, et al. Experimental, Systems, and Computational Approaches to Understanding the MicroRNA-Mediated Reparative Potential of Cardiac Progenitor Cell-Derived Exosomes From Pediatric Patients. Circ Res. 2017;120(4):701-712. [10] LV B, LI F, FANG J, et al. Hypoxia inducible factor 1α promotes survival of mesenchymal stem cells under hypoxia. Am J Transl Res. 2017;9(3): 1521-1529. [11] LI ZL, LV LL, TANG TT, et al. HIF-1α inducing exosomal microRNA-23a expression mediates the cross-talk between tubular epithelial cells and macrophages in tubulointerstitial inflammation. Kidney Int. 2019;95(2): 388-404. [12] ZHANG W, ZHOU X, YAO Q, et al. HIF-1-mediated production of exosomes during hypoxia is protective in renal tubular cells.Am J Physiol Renal Physiol. 2017;313(4):F906-F913. [13] ERDBRÜGGER U, LE TH. Extracellular Vesicles in Renal Diseases: More than Novel Biomarkers? J Am Soc Nephrol. 2016;27(1):12-26. [14] KARPMAN D, STÅHL AL, ARVIDSSON I. Extracellular vesicles in renal disease. Nat Rev Nephrol. 2017;13(9):545-562. [15] CHEN X, ZHOU J, LI X, et al. Exosomes derived from hypoxic epithelial ovarian cancer cells deliver microRNAs to macrophages and elicit a tumor-promoted phenotype. Cancer Lett. 2018;435:80-91. [16] ZHANG Y, HAO Z, WANG P, et al. Exosomes from human umbilical cord mesenchymal stem cells enhance fracture healing through HIF-1α-mediated promotion of angiogenesis in a rat model of stabilized fracture. Cell Prolif. 2019;52(2):e12570. [17] VALADI H, EKSTRÖM K, BOSSIOS A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654-659. [18] SKOG J, WÜRDINGER T, VAN RIJN S, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10(12):1470-1476. [19] SMYTHIES J, EDELSTEIN L. Transsynaptic modality codes in the brain: possible involvement of synchronized spike timing, microRNAs, exosomes and epigenetic processes. Front Integr Neurosci. 2013;6:126. [20] TAYLOR DD, GERCEL-TAYLOR C. The origin, function, and diagnostic potential of RNA within extracellular vesicles present in human biological fluids. Front Genet. 2013;4:142. [21] XU D, TAHARA H. The role of exosomes and microRNAs in senescence and aging. Adv Drug Deliv Rev. 2013;65(3):368-375. [22] HAN Q, LV L, WEI J, et al. Vps4A mediates the localization and exosome release of β-catenin to inhibit epithelial-mesenchymal transition in hepatocellular carcinoma. Cancer Lett. 2019;457:47-59. [23] CHEN W, QUAN Y, FAN S, et al. Exosome-transmitted circular RNA hsa_circ_0051443 suppresses hepatocellular carcinoma progression. Cancer Lett. 2020;475:119-128. [24] WU DM, WEN X, HAN XR, et al. Bone Marrow Mesenchymal Stem Cell-Derived Exosomal MicroRNA-126-3p Inhibits Pancreatic Cancer Development by Targeting ADAM9. Mol Ther Nucleic Acids. 2019;16:229-245. [25] YUE X, LAN F, XIA T. Hypoxic Glioma Cell-Secreted Exosomal miR-301a Activates Wnt/β-catenin Signaling and Promotes Radiation Resistance by Targeting TCEAL7. Mol Ther. 2019;27(11):1939-1949. [26] WANG D, WANG X, SI M, et al. Exosome-encapsulated miRNAs contribute to CXCL12/CXCR4-induced liver metastasis of colorectal cancer by enhancing M2 polarization of macrophages. Cancer Lett. 2020;474:36-52. [27] KALRA H, GANGODA L, FONSEKA P, et al. Extracellular vesicles containing oncogenic mutant β-catenin activate Wnt signalling pathway in the recipient cells. J Extracell Vesicles. 2019;8(1):1690217. [28] CHEN F, CHEN J, YANG L, et al. Extracellular vesicle-packaged HIF-1α-stabilizing lncRNA from tumour-associated macrophages regulates aerobic glycolysis of breast cancer cells. Nat Cell Biol. 2019;21(4):498-510. [29] WU G, ZHOU W, LIN X, et al. circRASSF2 Acts as ceRNA and Promotes Papillary Thyroid Carcinoma Progression through miR-1178/TLR4 Signaling Pathway. Mol Ther Nucleic Acids. 2020;19:1153-1163. [30] WU G, ZHOU W, PAN X, et al. Circular RNA Profiling Reveals Exosomal circ_0006156 as a Novel Biomarker in Papillary Thyroid Cancer. Mol Ther Nucleic Acids. 2020;19:1134-1144. [31] LIU J, FAN L, YU H, et al. Endoplasmic Reticulum Stress Causes Liver Cancer Cells to Release Exosomal miR-23a-3p and Up-regulate Programmed Death Ligand 1 Expression in Macrophages. Hepatology. 2019;70(1):241-258. [32] HAM S, LIMA LG, CHAI EPZ, et al. Breast Cancer-Derived Exosomes Alter Macrophage Polarization via gp130/STAT3 Signaling. Front Immunol. 2018; 9:871. [33] WANG J, YANG X, LI R, et al. Long non-coding RNA MYU promotes prostate cancer proliferation by mediating the miR-184/c-Myc axis. Oncol Rep. 2018; 40(5):2814-2825. [34] GAO L, MEI S, ZHANG S, et al. Cardio-renal Exosomes in Myocardial Infarction Serum Regulate Proangiogenic Paracrine Signaling in Adipose Mesenchymal Stem Cells. Theranostics. 2020;10(3):1060-1073. [35] WANG SY, LI Y, JIANG YS, et al. Investigation of serum miR-411 as a diagnosis and prognosis biomarker for non-small cell lung cancer. Eur Rev Med Pharmacol Sci. 2017;21(18):4092-4097. [36] TONG Y, YE C, REN XS, et al. Exosome-Mediated Transfer of ACE (Angiotensin-Converting Enzyme) From Adventitial Fibroblasts of Spontaneously Hypertensive Rats Promotes Vascular Smooth Muscle Cell Migration. Hypertension. 2018;72(4):881-888. [37] XU F, ZHONG JY, LIN X, et al. Melatonin alleviates vascular calcification and ageing through exosomal miR-204/miR-211 cluster in a paracrine manner. J Pineal Res. 2020;68(3):e12631. [38] GUPTA R, RADICIONI G, ABDELWAHAB S, et al. Intercellular Communication between Airway Epithelial Cells Is Mediated by Exosome-Like Vesicles. Am J Respir Cell Mol Biol. 2019;60(2):209-220. [39] JIANG K, YANG J, GUO S, et al. Peripheral Circulating Exosome-Mediated Delivery of miR-155 as a Novel Mechanism for Acute Lung Inflammation. Mol Ther. 2019;27(10):1758-1771. [40] 刘超.血清来源的外泌体对BEAS-2B细胞气道炎症相关因子表达和TLR4/NF-κB炎症信号通路的影响[D]. 南昌:南昌大学,2019. [41] WANG Y, ZHENG F, GAO G, et al. MiR-548a-3p regulates inflammatory response via TLR4/NF-κB signaling pathway in rheumatoid arthritis. J Cell Biochem. 2019;120(2): 1133-1140. [42] CHEN CW, FU M, DU ZH, et al. Long Noncoding RNA MRPL23-AS1 Promoteoid Cystic Carcinoma Lung Metastasis. Cancer Res. 2020 Feb 25. doi: 10.1158/0008-5472.CAN-19-0819. Online ahead of print. [43] 吴锋,王翠香,田华.肝细胞癌患者血清外泌体LncRNAZFAS1表达临床意义[J].中华肿瘤防治杂志, 2019,26(12):849-854. [44] HUANG L, WANG Y, CHEN J, et al. Long noncoding RNA PCAT1, a novel serum-based biomarker, enhances cell growth by sponging miR-326 in oesophageal squamous cell carcinoma. Cell Death Dis. 2019;10(7):513. [45] HU D, ZHAN Y, ZHU K, et al. Plasma Exosomal Long Non-Coding RNAs Serve as Biomarkers for Early Detection of Colorectal Cancer. Cell Physiol Biochem. 2018;51(6):2704-2715. [46] 张好良,蔡晨晨,张苗苗.血清外泌体源性长链非编码RNA RPN2-4在胃癌中的表达及其筛查价值[J]. 临床检验杂志, 2019,37(5):331-333. [47] SU YY, SUN L, GUO ZR, et al. Upregulated expression of serum exosomal miR-375 and miR-1307 enhance the diagnostic power of CA125 for ovarian cancer. J Ovarian Res. 2019;12(1):6. [48] ZHENG M, HOU L, MA Y, et al. Exosomal let-7d-3p and miR-30d-5p as diagnostic biomarkers for non-invasive screening of cervical cancer and its precursors. Mol Cancer. 2019;18(1):76. [49] 魏萍,杜鲁涛,王卿,等.血清外泌体miR-20b-5p对非小细胞肺癌的诊断价值[J].山东大学学报(医学版), 2019, 57(4):91-96. [50] MIN L, ZHU S, CHEN L, et al. Evaluation of circulating small extracellular vesicles derived miRNAs as biomarkers of early colon cancer: a comparison with plasma total miRNAs. J Extracell Vesicles. 2019;8(1):1643670. [51] SHAO N, XUE L, WANG R, et al. miR-454-3p Is an Exosomal Biomarker and Functions as a Tumor Suppressor in Glioma. Mol Cancer Ther. 2019; 18(2):459-469. [52] 张海涛,林文勇 解曼曼.冠心病患者外周血外泌体中microRNA基因芯片的差异性表达[J]. 临床心血管病杂志, 2019,35(6):501-505. [53] LI H, LIAO Y, GAO L, et al. Coronary Serum Exosomes Derived from Patients with Myocardial Ischemia Regulate Angiogenesis through the miR-939-mediated Nitric Oxide Signaling Pathway. Theranostics. 2018;8(8): 2079-2093. [54] YANG J, YU XF, LI YY, et al. Decreased HSP70 expression on serum exosomes contributes to cardiac fibrosis during senescence. Eur Rev Med Pharmacol Sci. 2019;23(9):3993-4001. [55] 宋晓菲,徐元宏.丙型肝炎病毒感染患者血清外泌体miR-122的表达量及临床意义探讨[J]. 安徽医科大学学报, 2019, 54(1):131-134. [56] FENG Y, LV LL, WU WJ, et al. Urinary Exosomes and Exosomal CCL2 mRNA as Biomarkers of Active Histologic Injury in IgA Nephropathy. Am J Pathol. 2018;188(11):2542-2552. [57] LIAO TL, HSIEH SL, CHEN YM, et al. Rituximab May Cause Increased Hepatitis C Virus Viremia in Rheumatoid Arthritis Patients Through Declining Exosomal MicroRNA-155. Arthritis Rheumatol. 2018;70(8):1209-1219. |
| [1] | Pu Rui, Chen Ziyang, Yuan Lingyan. Characteristics and effects of exosomes from different cell sources in cardioprotection [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 1-. |
| [2] | Lin Qingfan, Xie Yixin, Chen Wanqing, Ye Zhenzhong, Chen Youfang. Human placenta-derived mesenchymal stem cell conditioned medium can upregulate BeWo cell viability and zonula occludens expression under hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 4970-4975. |
| [3] | Jiang Yong, Luo Yi, Ding Yongli, Zhou Yong, Min Li, Tang Fan, Zhang Wenli, Duan Hong, Tu Chongqi. Von Mises stress on the influence of pelvic stability by precise sacral resection and clinical validation [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1318-1323. |
| [4] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [5] | Zhang Chao, Lü Xin. Heterotopic ossification after acetabular fracture fixation: risk factors, prevention and treatment progress [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1434-1439. |
| [6] | Zhou Jihui, Li Xinzhi, Zhou You, Huang Wei, Chen Wenyao. Multiple problems in the selection of implants for patellar fracture [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1440-1445. |
| [7] | Wang Debin, Bi Zhenggang. Related problems in anatomy mechanics, injury characteristics, fixed repair and three-dimensional technology application for olecranon fracture-dislocations [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1446-1451. |
| [8] | Gu Xia, Zhao Min, Wang Pingyi, Li Yimei, Li Wenhua. Relationship between hypoxia inducible factor 1 alpha and hypoxia signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1284-1289. |
| [9] | Ji Zhixiang, Lan Changgong. Polymorphism of urate transporter in gout and its correlation with gout treatment [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1290-1298. |
| [10] | Yuan Mei, Zhang Xinxin, Guo Yisha, Bi Xia. Diagnostic potential of circulating microRNA in vascular cognitive impairment [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1299-1304. |
| [11] | Zhang Xiumei, Zhai Yunkai, Zhao Jie, Zhao Meng. Research hotspots of organoid models in recent 10 years: a search in domestic and foreign databases [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1249-1255. |
| [12] | Liu Cong, Liu Su. Molecular mechanism of miR-17-5p regulation of hypoxia inducible factor-1α mediated adipocyte differentiation and angiogenesis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1069-1074. |
| [13] | Wang Xianyao, Guan Yalin, Liu Zhongshan. Strategies for improving the therapeutic efficacy of mesenchymal stem cells in the treatment of nonhealing wounds [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1081-1087. |
| [14] | Wan Ran, Shi Xu, Liu Jingsong, Wang Yansong. Research progress in the treatment of spinal cord injury with mesenchymal stem cell secretome [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1088-1095. |
| [15] | Liao Chengcheng, An Jiaxing, Tan Zhangxue, Wang Qian, Liu Jianguo. Therapeutic target and application prospects of oral squamous cell carcinoma stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1096-1103. |
| Viewed | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
Full text 196
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
Abstract 532
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||