Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (3): 452-457.doi: 10.12307/2023.884
Previous Articles Next Articles
Function and advantages of magnetically responsive hydrogel in bone tissue engineering
Chen Pinrui1, 2, Pei Xibo1, 2, Xue Yiyuan2, 3
- 1Department of Prosthodontics, West China Hospital of Stomatology, Sichuan University, Chengdu 610041, Sichuan Province, China; 2National Key Laboratory of Oral Disease Research, National Oral Disease Clinical Medical Center, Chengdu 610041, Sichuan Province, China; 3Jinjiang Clinic of West China Stomatological Hospital of Sichuan University, Chengdu 610041, Sichuan Province, China
-
Received:2022-12-14Accepted:2023-01-03Online:2024-01-28Published:2023-07-10 -
Contact:Xue Yiyuan, MD, Attending physician, National Key Laboratory of Oral Disease Research, National Oral Disease Clinical Medical Center, Chengdu 610041, Sichuan Province, China; Jinjiang Clinic of West China Stomatological Hospital of Sichuan University, Chengdu 610041, Sichuan Province, China -
About author:Chen Pinrui, Master candidate, Department of Prosthodontics, West China Hospital of Stomatology, Sichuan University, Chengdu 610041, Sichuan Province, China; National Key Laboratory of Oral Disease Research, National Oral Disease Clinical Medical Center, Chengdu 610041, Sichuan Province, China -
Supported by:the National Natural Science Foundation of China, No. 82271016 (to PXB); the Natural Science Foundation of Sichuan Province, No. 2022NSFSC1364 (to XYY)
CLC Number:
Cite this article
Chen Pinrui, Pei Xibo, Xue Yiyuan. Function and advantages of magnetically responsive hydrogel in bone tissue engineering[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(3): 452-457.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
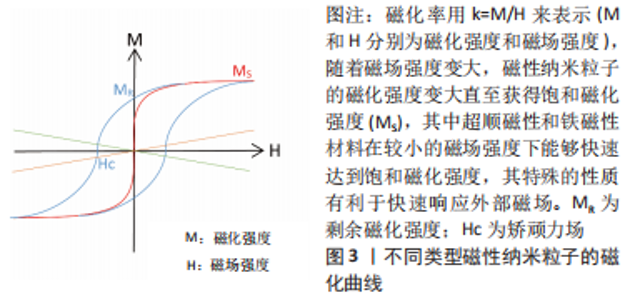
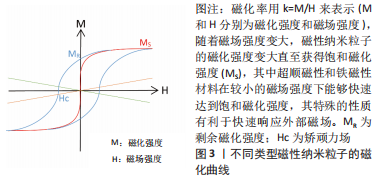
2.1 磁性纳米粒子概述 磁性纳米粒子是一种尺寸在100 nm以内的磁性颗粒,以铁和铁系氧化物居多,例如γ-Fe2O3和Fe3O4。磁性纳米粒子不仅具有纳米粒子的大多数优点,还兼具良好的导热性和导电性,目前已被广泛应用于药物传递、磁热疗、核磁成像及光热治疗等领域[5],近年来磁性纳米粒子在组织工程与再生医学领域受到了大量的关注。LEE等[6]将氧化铁纳米粒子注入梗死心脏,结果表明磁引导下磁性纳米粒子减少细胞凋亡和纤维化,促进血管生成和心脏功能恢复。FERNANDES等[7]发现磁性纳米粒子的加入使支架具有局部磁机械和磁电响应,在磁刺激下为细胞提供机械和电信号,有利于小鼠成骨前体细胞的黏附和增殖,另外,磁刺激进一步提高细胞活力,促进细胞向支架内生长。QIAN等[8]研究磁性纳米粒子对骨髓间充质干细胞的影响,结果表明,磁性纳米粒子以20 pg/细胞的浓度累积在骨髓间充质干细胞中,对其细胞增殖和分化无不良影响,而在外加磁场作用下,磁性纳米粒子可显著促进骨髓间充质干细胞增殖。此外,YANG等[9]在体外诱导骨髓间充质干细胞 21 d后,发现磁性纳米粒子在磁场的作用下具有诱导其成骨分化、成脂分化和成软骨分化的能力。因此,以下部分将回顾制备磁性纳米粒子的合成方法,磁性纳米粒子在骨组织工程应用所需的基本特性以及磁性纳米粒子的生物相容性。 2.1.1 磁性纳米粒子的合成方法 常用的合成方法有溶胶-凝胶法、溶剂热法、共沉淀法和热分解法。 (1)溶胶-凝胶法:是基于分子磁性前体在溶液中的羟基化,然后通过缩合和无机聚合得到磁性纳米粒子,通常需要额外的加热步骤才能获得最终的结晶状态[10]。 (2)溶剂热法:通常是在高压(> 100 kPa)和低温下(高于溶剂的沸点)进行,其中水热法是指采用水作为反应体系合成磁性纳米粒子,该方法提高了磁性纳米粒子在水介质中的稳定性。然而,水热法尚不能合成具有亲水性的高质量小尺寸磁性纳米粒子[11]。 (3)共沉淀法:是通过加碱而使亚铁盐和铁盐共沉淀,得到的磁性纳米粒子是水溶性的,该方法简单方便,是制备低毒性、高质量磁性纳米粒子的常用方法[12]。为提高磁性纳米粒子的稳定性,KIM等[13]通过乳化法在磁性纳米粒子表面包裹一层二氧化硅外壳,而LIU等[14]通过将磁性纳米粒子浸泡在酶缓冲液中,在其表面快速生长纯蛋白质外壳,得到具有抗机械、化学降解的涂层。 (4)热分解法:是在受控条件和高温下还原金属前驱体,得到的磁性纳米粒子具有高度单分散性[15]。该方法能够控制其尺寸、晶体结构和磁性,是设计出具有良好磁响应磁性纳米粒子的首选途径。 2.1.2 磁性纳米粒子的基本特性 磁性纳米粒子具备的基本特性如图3及表1所示,其中铁磁性磁性纳米粒子最为常见,对外界有强大的磁响应性[16]。有研究表明,仅含有少量铁磁性物质的骨组织工程支架,就能够对外界磁刺激迅速做出反应[17]。SHOKROLLAHI[18]进一步发现,在低于临界尺寸时,磁性纳米粒子会进入超顺磁性体系,当施加磁场时,磁性纳米粒子可以迅速达到饱和磁化强度,而移除磁场时,没有剩余的磁矩,这对于磁响应水凝胶的体内应用是至关重要的,因为剩余磁矩会导致磁性纳米粒子聚集。此外,多项研究显示理想的磁性纳米粒子超顺磁性行为的尺寸在100 nm以内[19-20]。"

抗磁材料容易产生电流,抵消外加磁场造成的影响而表现出抗磁性。尽管抗磁材料通常被认为是无磁性的,但它们可以响应高强度磁场,如抗磁棒状磁性纳米粒子会垂直于磁场排列,因此与水凝胶结合也有利于获得各向异性磁响应水凝胶[21]。 2.1.3 磁性纳米粒子的生物相容性 在生物应用层面,磁性纳米粒子在磁场作用下先沉积在细胞膜表面,然后通过内吞作用被细胞吞噬,进而影响细胞的生理功能[22],随着水凝胶的降解,磁性纳米粒子可以扩散到整个机体,因此,有必要研究磁性纳米粒子从水凝胶释放后的体内去向以及降解产物对机体的影响。 有研究表明,低剂量的磁性纳米粒子是安全的,可以在体内通过网状内皮系统、肝脏等已知的铁代谢途径被迅速消除[23],还可以通过电离同化成铁离子参与铁稳态,已被美国食品药物监督管理局批准应用于治疗缺铁性贫血、磁共振成像等[24]。一般来说,在血液中循环的磁性纳米粒子通常被过滤,部分被肝脏和脾脏巨噬细胞降解,然后通过胃肠道排出。具体而言,用于口服给药的磁性纳米粒子的清除途径取决于颗粒大小,颗粒小于10-15 nm的颗粒离开血液后可被肾脏迅速清除,小于500 nm的颗粒可被肝脏清除,而颗粒大于5 μm的颗粒可通过淋巴引流清除。过量的磁性纳米粒子倾向于积聚在其他富含巨噬细胞的组织中,如肺和脂肪组织[25-26]。基于细胞对铁离子具有高度耐受性,磁响应水凝胶中氧化铁的磁性纳米粒子在很长一段时间内被认为是相对安全的。"


2.2 磁响应水凝胶的制备策略 2.2.1 磁响应水凝胶的制备方法 关于磁响应水凝胶的常用制备方法主要包括以下3种: (1)混合法:磁性纳米粒子大多采用传统的共沉淀法制备,然后将制备的磁性纳米粒子沉淀物分散在水相或油相中形成铁液,避免其氧化和团聚。最后将铁液和水凝胶前驱体溶液的混合物进行交联,将磁性纳米粒子包覆在水凝胶中。SHI等[27]将双膦酸盐改性的透明质酸溶液与磁性纳米粒子混合,构建一种新型磁响应水凝胶,结果表明该水凝胶具有良好的生物相容性,并能够在体内缓慢降解。混合法制备的磁响应水凝胶,具有以下优点:首先,由于磁性纳米粒子的制备和交联是分开进行的,所以制备过程相对简单;其次,通过改变搅拌速度、控制铁液浓度和制备周期,可以在水凝胶中获得尺寸均匀的磁性纳米粒子。然而,混合法无法精确控制磁性纳米粒子在水凝胶中的分布。 (2)原位沉淀法:在原位沉淀过程中,无机盐中的铁离子在水凝胶网络中与碱性溶液发生水化反应生成磁响应水凝胶[28]。具体而言,首先通过聚合、变温或交联化反应制备水凝胶,然后将含有铁(Ⅲ)和铁(Ⅱ)的磁铁矿前驱体按1∶2的摩尔比添加到水凝胶中,得到均匀混合液,最后将混合物浸泡在碱性溶液中,诱导磁铁矿结晶的形成。ARIAS等[29]通过原位沉淀法设计了一种磁性细菌纤维素水凝胶,该凝胶可在饱和磁化条件下活化,并且在外加磁场刺激下具有较好的细胞相容性。原位沉淀法可以将多种无机物质应用于水凝胶网络的制备,并使其在水凝胶基质中具有良好的分散性。然而,制备过程中使用的碱性溶液可能会破坏水凝胶网络并对细胞有毒性作用,因此只适用于具有稳定结构且不包埋细胞的水凝胶网络。 (3)共价结合法:磁性纳米粒子和水凝胶网络之间形成共价键。具体而言,将磁性纳米粒子通过表面官能团,作为交联剂与水凝胶单体形成共价键。HU等[30]将甲基丙烯酸三甲氧基丙酯接枝Fe3O4纳米粒子,与无毒的聚丙烯酰胺水凝胶共价结合,制备出一种具有较高力学性能的磁响应水凝胶。共价结合法在一定程度上提高了磁响应水凝胶的稳定性,降低其漏磁风险,改善其机械性能。然而该方法存在制作时间长、成本高、工艺复杂等缺点。 2.2.2 各向异性磁响应水凝胶的设计策略 在以往研究中,冷冻法、纳米填料法、剪切诱导法、微凝胶单元自组装法已被用于制备各向异性水凝胶[31-34],然而构建出实现药物受控递送的各向异性水凝胶仍具挑战性。在水凝胶中加入磁响应纤维或棒状微粒等抗磁材料可构建出具有天然骨组织状的各向异性水凝胶,并在外加磁场远程驱动下调控药物释放及细胞行为[35-38]。DE FRANCE等[39]制备了一种用磁响应纤维素纳米晶体改性的可注射聚乙二醇水凝胶,研究发现1.2 T的磁场强度足以在凝胶化过程中诱导纳米晶体的排列,形成具有方向依赖性机械特性的各向异性水凝胶,并促进包裹的骨骼肌成肌细胞的排列和分化。然而,抗磁材料需高强度磁场响应,不符合骨组织工程的生物学需求。而磁性纳米粒子作为具有高度磁响应性的元件,能够在最低强度磁刺激下发挥功能,是设计各向异性磁响应水凝胶的首选策略。具体方法为,磁性纳米粒子和水凝胶分别制备后,通过磁性纳米粒子的溶液与水凝胶溶液混合进行交联,将磁性纳米粒子封装到水凝胶中。FARZANEH等[40]采用共沉淀法制备钴铁氧体纳米颗粒,将前驱体溶液与水凝胶混合得到一种具有促进人牙髓干细胞增殖和成骨分化潜能的磁响应凝胶。另外,在最近的研究中微流体也被用于生产这些各向异性元件,WANG等[41]制备了一种磁性Fe3O4微球-明胶水凝胶,研究结果显示,20 mT低浓度Fe3O4纳米粒子显著促进小鼠成骨前体细胞增殖。"

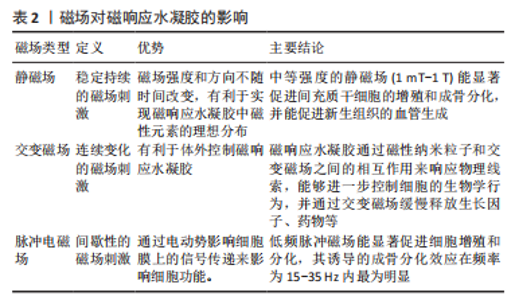
2.3 磁响应水凝胶在骨组织工程的应用 为了更好地将磁性纳米粒子整合到水凝胶中应用于骨组织工程,还需满足以下要求: (1)磁性纳米粒子应在体液甚至在血液中保持稳定,以避免其释放后而发生团聚。 (2)磁性纳米粒子应表现出无毒行为,最好有表面涂层以避免金属离子的泄漏,对包裹在水凝胶中的细胞不会造成不良影响,若植入体内释放并进入循环,还需要有良好的组织相容性。在磁响应水凝胶中使用低剂量的磁性纳米粒子是相对安全的,但还需尽可能地控制磁性纳米粒子的降解,以确保在长期外加磁场下发挥远程磁响应性的作用。 (3)使用对磁场高度敏感的磁性纳米粒子,以减少磁性材料的含量和调控所需磁辐射的强度,将相关的潜在毒性和安全风险降至最低,进一步在低强度外部磁场下设计磁响应水凝胶,相关磁场对磁响应水凝胶的影响见表2。"


2.3.1 磁响应水凝胶作为骨组织工程的支架 开发更加高效的新型支架一直是骨组织工程的主要研究方向,磁响应水凝胶不仅可以模拟细胞外基质的动态力学特性,还具有可注射性,能够填充不规则骨缺损部位。PETRETTA等[42]构建一种由胶原制成的磁响应水凝胶支架,其中嵌入纳米羟基磷灰石和超顺磁性氧化铁纳米粒子(superparamagnetic iron oxide nanoparticles,SPIONs),结果表明该支架上接种的间充质干细胞显示出良好的细胞增殖和成骨潜能。HUANG等[43]将SPIONs和羟基磷灰石纳米晶体加入到基于二嵌段共聚物的热响应可注射水凝胶中,并将小鼠成骨前体细胞包裹在水凝胶支架中,结果表明磁性纳米粒子的加入提高了水凝胶的复黏度,值得注意的是在静磁场和交变磁场作用下均观察到磁性纳米粒子组的碱性磷酸酶、骨钙素、骨桥蛋白表达显著增加。FILIPPI等[4]设计一种磁性纳米粒子改性的聚乙二醇水凝胶支架,结果发现与单独的磁性纳米粒子相比,磁性纳米粒子改性后的水凝胶支架显著增强碱性磷酸酶活性,上调成骨相关基因的表达,促进矿化及血管形成;另外,水凝胶支架通过模拟细胞外基质微环境能够进一步调控细胞行为。 ABDEEN等[44]将羰基铁颗粒加入到聚丙烯酰胺水凝胶中,利用交变磁场(?1.0 T至1.0 T)调节水凝胶支架硬度,结果显示,随着磁场的变化基质硬度是可逆的,水凝胶与间充质干细胞共培养10 d后Runt相关转录因子2表达水平上调,并且学者发现在坚硬基质上培养的间充质干细胞更倾向于成骨分化,这意味着早期机械转导可能会促使成骨信号的启动。HUANG等[45]将SPIONs接枝在胶原纳米纤维上,构建了一种能够及时控制巨噬细胞极化的超顺磁性水凝胶,研究发现水凝胶经磁化后能够诱导巨噬细胞M1型向M2型转变,进而促进体外骨髓间充质干细胞的成骨分化;通过调节在磁场下的暴露时间,水凝磁响应水凝胶在损伤初期能够使M1型巨噬细胞充分浸润,并在炎症后期使M1型巨噬细胞快速转化为M2型巨噬细胞,从而在体内获得最佳的成骨效果。在机制上,超顺磁水凝胶在磁场作用下可以激活巨噬细胞的Podosome/Rho/ROCK机械信号通路[45]。 综上所述,氧化铁纳米粒子的加入改善了磁响应水凝胶的力学性能,使其具有较高的强度和刚度,有利于骨细胞的增殖和黏附,然而磁驱动刺激与骨细胞之间增强骨形成的的潜在机制仍在探索中。 2.3.2 基于磁响应水凝胶的三维微环境培养平台 大多数体外细胞、组织培养系统是基于2D底物(如培养皿及多孔板),这与体内细胞的3D微环境有很大不同。为了探究2D、3D培养条件的差异,CARVALHO等[46]制备一种由聚乳酸和钴铁氧体微球组成的磁响应水凝胶,研究表明与2D基质相比,在3D基质上培养的小鼠成骨前体细胞的增殖速率明显增加。PARFENOV等[47]研究发现,细胞在磁刺激下可以被驱动到三维支架的中心,这对促进骨组织再生和愈合具有重要意义。另外YUAN等[48]将磁性纳米粒子嵌入到天然胶原蛋白水凝胶中,制备了一种三维磁响应水凝胶,在静磁场刺激下,该磁响应水凝胶促进人成骨肉瘤细胞的增殖、加快碱性磷酸酶形成及矿化,提高Runt相关转录因子2、骨形成发生蛋白2和骨形成发生蛋白4等成骨相关基因表达。HAN等[49]将牙髓间充质干细胞通过自组装方式形成微球,获得了3D磁响应水凝胶培养环境,结果显示牙髓间充质干细胞球状体比经典2D培养的牙髓间充质干细胞表现出更明显的成骨分化倾向。此外有研究显示,3D环境下磁响应水凝胶与细胞之间的界面磁力能够激活细胞表面敏感受体、增强细胞活性、促进新生骨质与宿主骨的整合[50]。目前关于活体的3D微组织工程尚未成熟[51],未来还需进一步研究。 2.3.3 基于磁响应水凝胶的载药控释体系 近年来,人们一直致力于优化药物释放系统,减少药物不良反应和毒性以提高疗效。DENYER等[52]发现在外部磁场的引导下能够将磁性纳米粒子靶向到特定部位,这意味着将药物分子负载到磁性纳米粒子上是一种简单、直接的磁性靶向给药途径。磁响应水凝胶可以将载药磁性纳米粒子预载到支架上,通过磁场控制实现药物的控释,提高运载效率。SILVA等[53]制备了含甲基丙烯酸化硫酸软骨素、血小板裂解物和磁性纳米粒子的磁响应水凝胶,将人脂肪来源干细胞和人肌腱来源细胞包埋于水凝胶中,并对其生物学性能进行评价。值得一提的是,该水凝胶具有骨和肌腱的三维梯度结构,在没有磁场的情况下20 d内不发生降解且活性良好,当施加磁场刺激后,磁性水凝胶显示出降解,随着支架的降解,血小板裂解物相关生长因子表达上调,验证了磁场刺激能够调控药物的释放[53]。另外,LI等[54]通过接枝的方法将载骨形态发生蛋白2的糖基化SPIONs梯度嵌入至琼脂糖凝胶中,体外结果显示培养28 d后骨形态发生蛋白2被稳定封装在糖基化SPIONs中,并且能够在磁场下持续缓慢释放,同时观察到包裹人间充质干细胞的琼脂糖水凝胶上形成磁性图案,以及碱性磷酸酶、骨桥蛋白表达上调和密集的组织矿化,结果表明,该磁性梯度水凝胶具有良好的控释体系,并能够发挥成骨的作用。MADANI等[55]设计了一种磁响应水凝胶给药系统,在外层的凝胶室装载了基质细胞衍生因子1α,用于快速建立间充质干细胞的3D培养,而内层的磁性纳米粒子凝胶室装载了骨形态发生蛋白2,可根据磁刺激的时间即刻或延迟递送骨形态发生蛋白2。结果表明,外膜室能够募集并容纳间充质干细胞,而基质细胞衍生因子1α可以进一步增强募集间充质干细胞的速度;内膜室可以通过远程磁刺激控制骨形态发生蛋白2的释放,缓释时间长达11 d。 总体而言,抗生素、生长因子以及与成骨相关DNA/RNA虽然可以改善骨缺损的修复效果,但是不能针对特定的部位或产生直接的修复作用。目前的大量实验结果证明,氧化铁纳米颗粒可以作为一种传递手段,在外部磁场的作用下能够有效地携带药物分子靶向特定的位置,并通过磁场开关控制药物的缓释。这种水凝胶给药系统在骨祖细胞募集和骨分化因子的释放时间上具有可控性,能够协同增强新骨生成,从而提高最终治疗效果。 2.3.4 磁响应水凝胶在骨组织工程的其他应用 (1)磁热疗:热疗癌症疗法,即将某些癌症器官或组织加热到41-46 ℃,已被证明是肿瘤治疗的有效方法。然而,精确地控制目标区域内外的温度仍然具有挑战性。最近,磁响应水凝胶已被应用于通过外部磁热疗远程加热目标肿瘤,同时从水凝胶中可控地释放抗癌药物。其中,磁性纳米粒子具有较高的光热转换效率,在适当的温度刺激下可以加快血液循环,调节骨代谢,促进新骨形成。为了探索磁热疗与骨再生的相关性,CAO等[56]设计的含有磁性纳米粒子的壳聚糖/聚乙二醇水凝胶,能在交变磁场作用下实现升温快速,具有良好的生物相容性。当温度为43 ℃时,该磁响应水凝胶中的间充质干细胞表现出最佳的细胞活力和最高的碱性磷酸酶活性。YUAN等[57]利用磁性纳米粒子和氧化石墨烯作为磁热剂和光热剂进行热疗,制备了一种磁光双响应水凝胶,实验结果表明,该水凝胶可以消除90%以上的肿瘤细胞,有效抑制肿瘤生长。WANG等[58]将铁酸钴-铁酸锰磁性纳米粒子聚合物包裹在琼脂糖凝胶中,构建了一种具有优良骨诱导和磁热性能的纳米颗粒-水凝胶复合材料,其在交变磁场下可以激发出温和的磁热疗(41-42 ℃)效应,并通过热休克蛋白90激活的PI3K/Akt通路,显著促进小鼠成骨前体细胞的成骨分化和生物矿化。综上,磁响应水凝胶系统为治疗骨癌切除后的组织重建提供了新的策略,尽管磁靶向作用能够使磁响应水凝胶存在于特定部位,但是关于磁性纳米粒子在体内的迁移、代谢去向仍然存在争议,未来有望进一步探索。 (2)生物成像:超小顺磁性氧化铁(ultra-small paramagnetic iron oxide,USPIO)作为一种具有显著对比度的造影剂,具有优良的理化性能和生物相容性,起到增强磁共振成像的效果,近年来在生物成像受到了极大关注。CHEN等[59]成功合成了一种明胶/明胶-聚己内酯/超小型顺磁性氧化铁/氧化石墨烯磁响应水凝胶,该材料具有良好的机械性能、MRI对比度、生物相容性和血液相容性,且能显著提高导电性能,增强体内电位的信号传输,有利于研究组织工程支架的降解和吸收,同时还能支持骨髓间充质干细胞的黏附,有效促进细胞的增殖黏附。LI等[60]也证实了USPIO能够实现MRI原位成像,监测支架在体内的降解情况,且有利于骨髓间充质干细胞在支架上的黏附。综上,USPIO标记的导电支架为骨相关的生物成像应用提供了一种切实可行的方法,能够提高磁共振成像的可见性,具有良好的临床应用前景。"

| [1] 付正,李润泽,罗皓天,等.自修复水凝胶在骨组织工程中的应用[J].中国组织工程研究,2022,26(16):2590-2595. [2] CUI Z, KIM S, BALJON JJ, et al. Microporous methacrylated glycol chitosan-montmorillonite nanocomposite hydrogel for bone tissue engineering. Nat Commun. 2019;10(1):3523. [3] ALFORD AI, KOZLOFF KM, HANKENSON KD. Extracellular matrix networks in bone remodeling. Int J Biochem Cell B. 2015;65:20-31. [4] FILIPPI M, DASEN B, GUERRERO J, et al. Magnetic nanocomposite hydrogels and static magnetic field stimulate the osteoblastic and vasculogenic profile of adipose-derived cells. Biomaterials. 2019;223:119468. [5] 沈梦杰,杨琨,刘琪.氧化铁纳米粒子在骨组织修复再生中的研究与应用[J].中国组织工程研究,2019,23(14):2248-2253. [6] LEE J, PARK B, KIM J, et al. Nanovesicles derived from iron oxide nanoparticles-incorporated mesenchymal stem cells for cardiac repair. Sci Adv. 2020;6(18):eaaz0952. [7] FERNANDES MM, CORREIA DM, RIBEIRO C, et al. Bioinspired three-dimensional magnetoactive scaffolds for bone tissue engineering. ACS Appl Mater Interfaces. 2019; 11(48):45265-45275. [8] QIAN W, QIAN M, WANG Y, et al. Combination glioma therapy mediated by a dual-targeted delivery system constructed using OMCN-PEG-Pep22/DOX. Small. 2018; 14(42):e1801905. [9] YANG F, LU J, KE Q, et al. Magnetic mesoporous calcium sillicate/chitosan porous scaffolds for enhanced bone regeneration and photothermal-chemotherapy of osteosarcoma. Sci Rep. 2018;8(1):7345. [10] ANSARI F, SOBHANI A, SALAVATI-NIASARI M. Simple sol-gel synthesis and characterization of new CoTiO3/CoFe2O4 nanocomposite by using liquid glucose, maltose and starch as fuel, capping and reducing agents. J Colloid Interface Sci. 2018; 514:723-732. [11] DAOU TJ, POURROY G, BÉGIN-COLIN S, et al. Hydrothermal synthesis of monodisperse magnetite nanoparticles. Chem Mater. 2006;18(18):4399-4404. [12] LIN S, LIN K, LU D, et al. Preparation of uniform magnetic iron oxide nanoparticles by co-precipitation in a helical module microchannel reactor. J Environ Chem Eng. 2017;5(1):303-309. [13] KIM H, KIM D, JEONG C, et al. Assembly of plasmonic and magnetic nanoparticles with fluorescent silica shell layer for tri-functional SERS-magnetic-fluorescence probes and its bioapplications. Sci Rep. 2018;8(1):13938. [14] LIU R, ZHAO J, HAN Q, et al. One‐step assembly of a biomimetic biopolymer coating for particle surface engineering. Adv Mater. 2018;30(38):e1802851. [15] UNNI M, UHL AM, SAVLIWALA S, et al. Thermal decomposition synthesis of iron oxide nanoparticles with diminished magnetic dead layer by controlled addition of oxygen. ACS Nano. 2017;11(2):2284-2303. [16] IWASA K, REDDI AH. Pulsed electromagnetic fields and tissue engineering of the joints. Tissue Eng Part B Rev. 2018;24(2):144-154. [17] LUO C, YANG X, LI M, et al. A novel strategy forin vivo angiogenesis and osteogenesis: magnetic micro-movement in a bone scaffold. Artif Cell Nanomed B. 2018;46(sup2):636-645. [18] SHOKROLLAHI H. Structure, synthetic methods, magnetic properties and biomedical applications of ferrofluids. Mat Sci Eng C-Mater. 2013;33(5):2476-2487. [19] KHAN I, SAEED K, KHAN I. Nanoparticles: Properties, applications and toxicities. Arab J Chem. 2019;12(7):908-931. [20] JIANG Z, SHAN K, SONG J, et al. Toxic effects of magnetic nanoparticles on normal cells and organs. Life Sci. 2019;220:156-161. [21] PETROV DA, ZAKHLEVNYKH AN. Statistical theory of magnetic field induced phase transitions in negative diamagnetic anisotropy liquid crystals doped with carbon nanotubes. J Mol Liq. 2019;287:110901. [22] THERUVATH AJ, NEJADNIK H, MUEHE AM, et al. Tracking cell transplants in femoral osteonecrosis with magnetic resonance imaging: a proof-of-concept study in patients. Clin Cancer Res. 2018;24(24):6223-6229. [23] AUERBACH M, CHERTOW GM, ROSNER M. Ferumoxytol for the treatment of iron deficiency anemia. Expert Rev Hematol. 2018;11(10):829-834. [24] VENTOLA CL. Progress in nanomedicine: approved and investigational nanodrugs. Pharmacol Therapeut. 2017;42(12):742-755. [25] SUN C, LEE JSH, ZHANG M. Magnetic nanoparticles in MR imaging and drug delivery. Adv Drug Delivery Rev. 2008;60(11):1252-1265. [26] ARAMI H, KHANDHAR A, LIGGITT D, et al. In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles. Chem Soc Rev. 2015;44(23):8576-8607. [27] SHI L, ZENG Y, ZHAO Y, et al. Biocompatible injectable magnetic hydrogel formed by dynamic coordination network. ACS Appl Mater Interfaces. 2019;11(49):46233-46240. [28] LIU K, HAN L, TANG P, et al. An Anisotropic hydrogel based on mussel-inspired conductive ferrofluid composed of electromagnetic nanohybrids. Nano Lett. 2019; 19(12):8343-8356. [29] ARIAS SL, SHETTY A, DEVORKIN J, et al. Magnetic targeting of smooth muscle cells in vitro using a magnetic bacterial cellulose to improve cell retention in tissue-engineering vascular grafts. Acta Biomater. 2018;77:172-181. [30] HU X, NIAN G, LIANG X, et al. Adhesive tough magnetic hydrogels with high Fe3O4 content. ACS Appl Mater Interfaces. 2019;11(10):10292-10300. [31] CHAU M, DE FRANCE KJ, KOPERA B, et al. Composite hydrogels with tunable anisotropic morphologies and mechanical properties. Chem Mater. 2016;28(10):3406-3415. [32] LU Q, BAI S, DING Z, et al. Hydrogel assembly with hierarchical alignment by balancing electrostatic forces. Adv Mater Interfaces. 2016;3(8):1500687. [33] WANG N, MA M, LUO Y, et al. Mesoporous silica nanoparticles-reinforced hydrogel scaffold together with pinacidil loading to improve stem cell adhesion. Chem Nano Mat. 2018;4(7):631-641. [34] LIN XY, WANG ZJ, PAN PJ, et al. Monodomain hydrogels prepared by shear-induced orientation and subsequent gelation. Rsc Adv. 2016;6(97):95239-95245. [35] OMIDINIA ANARKOLI A, BOESVELD S, TUVSHINDORJ U, et al. An injectable hybrid hydrogel with oriented short fibers induces unidirectional growth of functional nerve cells. Small. 2017;13(36):1702207. [36] ECHAVE MC, DOMINGUES RMA, GÓMEZ-FLORIT M, et al. Biphasic hydrogels integrating mineralized and anisotropic features for interfacial tissue engineering. ACS Appl Mater Interfaces. 2019;11(51):47771-47784. [37] ARAÚJO-CUSTÓDIO S, GOMEZ-FLORIT M, TOMÁS AR, et al. Injectable and magnetic responsive hydrogels with bioinspired ordered structures. ACS Biomater Sci Eng. 2019; 5(3):1392-1404. [38] ROSE J C, CÁMARA-TORRES M, RAHIMI K, et al. Nerve cells decide to orient inside an injectable hydrogel with minimal structural guidance. Nano Lett. 2017;17(6):3782-3791. [39] DE FRANCE KJ, YAGER KG, CHAN KJW, et al. Injectable anisotropic nanocomposite hydrogels direct in situ growth and alignment of myotubes. Nano Lett. 2017;17(10): 6487-6495. [40] FARZANEH S, HOSSEINZADEH S, SAMANIPOUR R, et al. Fabrication and characterization of cobalt ferrite magnetic hydrogel combined with static magnetic field as a potential bio-composite for bone tissue engineering. J Drug Deliv Sci Tec. 2021;64:102525. [41] WANG J, LI P, LI K, et al. The Effect of Magnetic Poly (lactic-co-glycolic acid) microsphere-gelatin hydrogel on the growth of pre-osteoblasts under static magnetic field. J Biomed Nanotechnol. 2020;16(11):1658-1666. [42] PETRETTA M, GAMBARDELLA A, DESANDO G, et al. Multifunctional 3D-printed magnetic polycaprolactone/hydroxyapatite scaffolds for bone tissue engineering. Polymers. 2021;13(21):3825. [43] HUANG WS, CHU IM. Injectable polypeptide hydrogel/inorganic nanoparticle composites for bone tissue engineering. PLoS One. 2019;14(1):e0210285. [44] ABDEEN AA, LEE J, BHARADWAJ NA, et al. Temporal modulation of stem cell activity using magnetoactive hydrogels. Adv Healthc Mater. 2016;5(19):2536-2544. [45] HUANG D, XU K, HUANG X, et al. Remotely temporal scheduled macrophage phenotypic transition enables optimized immunomodulatory bone regeneration. Small. 2022; 18(39):2203680. [46] CARVALHO EO, RIBEIRO C, CORREIA DM, et al. Biodegradable hydrogels loaded with magnetically responsive microspheres as 2D and 3D scaffolds. Nanomaterials. 2020;10(12):2421. [47] PARFENOV VA, MIRONOV VA, KOUDAN EV, et al. Fabrication of calcium phosphate 3D scaffolds for bone repair using magnetic levitational assembly. Sci Rep. 2020;10(1):4013. [48] YUAN Z, MEMARZADEH K, STEPHEN AS, et al. Development of a 3D collagen model for the In vitro evaluation of magnetic-assisted osteogenesis. Sci Rep. 2018;8(1):16270. [49] HAN X, TANG S, WANG L, et al. Multicellular spheroids formation on hydrogel enhances osteogenic/odontogenic differentiation of dental pulp stem cells under magnetic nanoparticles induction. Int J Nanomed. 2021;16:5101-5115. [50] PARDO A, GÓMEZ-FLORIT M, BARBOSA S, et al. Magnetic nanocomposite hydrogels for tissue engineering: design concepts and remote actuation strategies to control cell fate. ACS Nano. 2021;15(1):175-209. [51] MOK H, ZHANG M. Superparamagnetic iron oxide nanoparticle-based delivery systems for biotherapeutics. Expert Opin Drug Deliv. 2013;10(1):73-87. [52] DENYER S, BHIMANI AD, PAPASTEFAN S, et al. Magnetic kyphoplasty: a novel drug delivery system for the spinal column. PLOS One. 2018;13(7):e201402. [53] SILVA ED, BABO PS, COSTA-ALMEIDA R, et al. Multifunctional magnetic-responsive hydrogels to engineer tendon-to-bone interface. Nanomedicine. 2018;14(7):2375-2385. [54] LI C, ARMSTRONG JP, PENCE IJ, et al. Glycosylated superparamagnetic nanoparticle gradients for osteochondral tissue engineering. Biomaterials. 2018;176:24-33. [55] MADANI SZM, REISCH A, ROXBURY D, et al. A magnetically responsive hydrogel system for controlling the timing of bone progenitor recruitment and differentiation factor deliveries. ACS Biomater Sci Eng. 2020;6(3):1522-1534. [56] CAO Z, WANG D, LI Y, et al. Effect of nanoheat stimulation mediated by magnetic nanocomposite hydrogel on the osteogenic differentiation of mesenchymal stem cells. Sci China Life Sci. 2018;61(4):448-456. [57] YUAN P, YANG T, LIU T, et al. Nanocomposite hydrogel with NIR/magnet/enzyme multiple responsiveness to accurately manipulate local drugs for on-demand tumor therapy. Biomaterials. 2020;262:120357. [58] WANG L, HU P, JIANG H, et al. Mild hyperthermia-mediated osteogenesis and angiogenesis play a critical role in magnetothermal composite-induced bone regeneration. Nano Today. 2022;43:101401. [59] CHEN J, HU H, FENG L, et al. Preparation and characterization of 3D porous conductive scaffolds with magnetic resonance enhancement in tissue engineering. Biomed Mater. 2019;14(4):45013. [60] LI H, YIN Y, XIANG Y, et al. A novel 3D printing PCL/GelMA scaffold containing USPIO for MRI-guided bile duct repair. Biomed Mater. 2020;15(4):45004. |
| [1] | Gao Xueyu, Zhang Wentao, Sun Tianze, Zhang Jing, Li Zhonghai. Application of metal ions in bone tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(3): 439-444. |
| [2] | Long Zhirui, Huang Lei, Xiao Fang, Wang Lin, Wang Xiaobei. Characteristics of hydrogel microspheres in bone tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(3): 472-478. |
| [3] | Xu Jing, Lyu Huixin, Bao Xin, Zhang Yi, Wang Yihan, Zhou Yanmin. Application of near infrared responsive hydrogels in tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(3): 486-492. |
| [4] | Fan Yongjing, Wang Shu, Jin Wulong. Characteristics, advantages and application of osteogenic differentiation of jaw bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(1): 100-106. |
| [5] | Tang Haotian, Liao Rongdong, Tian Jing. Application and design of piezoelectric materials for bone defect repair [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 1117-1125. |
| [6] | Xu Yan, Li Ping, Lai Chunhua, Zhu Peijun, Yang Shuo, Xu Shulan. Piezoelectric materials for vascularized bone regeneration [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 1126-1132. |
| [7] | Qin Yuxing, Ren Qiangui, Li Zilong, Quan Jiaxing, Shen Peifeng, Sun Tao, Wang Haoyu. Action mechanism and prospect of bone microvascular endothelial cells for treating femoral head necrosis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 955-961. |
| [8] | Zhang Min, Zhang Xiaoming, Liu Tongbin. Application potential of naringin in bone tissue regeneration [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(5): 787-792. |
| [9] | Xiong Wei, Yuan Lingmei, Qian Guowen, Huang Jinyang, Pan Bin, Guo Ling, Zeng Zhikui. Value of a critical bone defect animal model in evaluating osteogenic efficacy of bone tissue engineering scaffold [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(35): 5714-5720. |
| [10] | Liu Zixuan, Li Yan, Ji Lin, Xia Delin. Biological properties of nano-hydroxyapatite-zinc oxide composite scaffolds and their effects on the behavior of MC3T3-E1 osteoblasts [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(34): 5441-5447. |
| [11] | Li Li, Li Xiao, Li Duchenhui, Zhang Jie, Xiao Tianjiao, Kang Jiabing, Tian Ai. Regulation of interleukin-4 on osteoclast differentiation during bone regeneration guided by bone replacement materials [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(34): 5455-5461. |
| [12] | Li Xinlun, Zhu Yushu, Yang Yiling, He Siqi, Wen Nan, Mu Yandong. Construction and in vivo osteogenesis of microspheres loaded with immunomodulatory peptide/miR-26a complexes for slow release [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(34): 5469-5476. |
| [13] | You Yan, Chen Jiawen, Lin Binbin, Wu Jingyi, Liu Peng, Wu Buling, Sun Tianyu. Mechanism and application of glycosaminoglycan in bone tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(34): 5538-5545. |
| [14] | Xu Zhengyi, Wan Qianbing, Chen Junyu. Natural small molecular compounds in the treatment of bone-related diseases by regulating type H blood vessels and its application in tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(34): 5546-5553. |
| [15] | Zhao Mingyue, Yang Shun, Tu Xiling, Gao Li, Yang Kun, Liu Qi. Application of platelet-rich plasma combined with electrospun nanoscaffolds in bone and soft tissue [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(34): 5554-5560. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||