Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (1): 100-106.doi: 10.12307/2023.790
Previous Articles Next Articles
Characteristics, advantages and application of osteogenic differentiation of jaw bone marrow mesenchymal stem cells
Fan Yongjing, Wang Shu, Jin Wulong
- Department of Stomatology, Affiliated Hospital of Inner Mongolia Medical University, Hohhot 010010, Inner Mongolia Autonomous Region, China
-
Received:2022-10-25Accepted:2023-01-18Online:2024-01-08Published:2023-06-28 -
Contact:Wang Shu, MD, Associate chief physician, Department of Stomatology, Affiliated Hospital of Inner Mongolia Medical University, Hohhot 010010, Inner Mongolia Autonomous Region, China Jin Wulong, MD, Chief physician, Department of Stomatology, Affiliated Hospital of Inner Mongolia Medical University, Hohhot 010010, Inner Mongolia Autonomous Region, China -
About author:Fan Yongjing, Master, Attending physician, Department of Stomatology, Affiliated Hospital of Inner Mongolia Medical University, Hohhot 010010, Inner Mongolia Autonomous Region, China -
Supported by:Youth Exploration Project of the Affiliated Hospital of Inner Mongolia Medical University, No. 2022NYFYTS014 (to FYJ)
CLC Number:
Cite this article
Fan Yongjing, Wang Shu, Jin Wulong. Characteristics, advantages and application of osteogenic differentiation of jaw bone marrow mesenchymal stem cells[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(1): 100-106.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
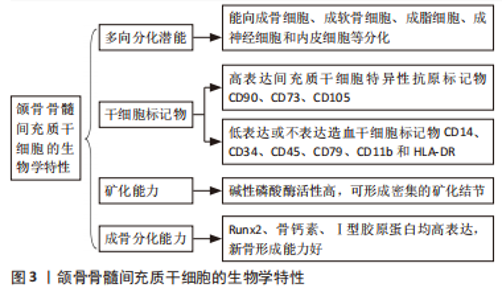
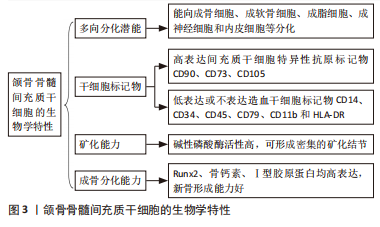
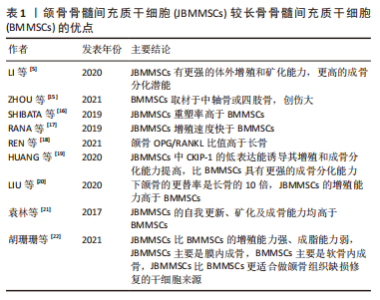
2.1 JBMMSCs的生物学特性 2005年,MATSUBARA等从人的牙槽骨中提取出JBMMSCs,发现其表面标志物、形态学特征与骨髓间充质干细胞非常相似,而且能向成骨细胞、成软骨细胞、成脂细胞、成神经细胞和成内皮细胞等分化。JBMMSCs可高表达间充质干细胞特异性抗原标记物CD90、CD73、CD105,而造血干细胞标记物CD14、CD34、CD45、CD79、CD11b和HLA-DR低表达或不表达[6-7]。JBMMSCs的成骨分化相关转录因子如成骨分化特异性转录因子Runx2(确定成骨分化开始的早期标志物)、骨钙素(晚期成骨细胞成熟的标志物)、Ⅰ型胶原蛋白(刺激成骨细胞中的钙和磷沉积,形成羟基磷灰石或骨矿物质)均高表达[8]。碱性磷酸酶是骨细胞形成不可缺少的酶,是早期评价和识别成骨细胞分化程度的重要指标,碱性磷酸酶活性反映成骨细胞的活性,实验证实JBMMSCs的碱性磷酸酶活性较高,而且经茜素红染色,JBMMSCs可被红染,并可见密集的矿化结节形成[7]。以上均证明JBMMSCs成骨分化的生物学特性良好,具有优良的成骨分化潜能。 有学者从大鼠下颌骨分离培养获得JBMMSCs,发现其具有较强的增殖和成骨分化能力,不仅能加速骨代谢,而且能促进骨折愈合,可作为颌骨组织工程的优质种子细胞[9]。Redondo等[10]取上颌骨根尖囊肿患者自体牙槽骨,将培养获得的JBMMSCs植入其骨缺损处,术后7个月后复查,CT扫描显示缺损处牙槽骨的骨密度明显增加,且无炎症或其他不良反应。有研究发现在牙髓炎根管治疗后不仅牙体硬组织形成,还有骨组织形成,探索原因发现是根管治疗时大量的JBMMSCs随血液进入根管内,并分化为成骨细胞,促进了根管内异位骨样组织沉积[11]。LUO等[12]进行比格犬体内实验,将获得的犬JBMMSCs植入比格犬的根分叉缺损处,发现根分叉区形成新骨和类骨质结构的效果较好。NAKAJIMA等[13]通过收集拔牙术后牙槽窝中的骨组织,经培养诱导获得JBMMSCs,将其植入到患者颌骨缺损处,观察一段时间后发现缺损处有新骨形成,且新骨密度较高,成骨分化相关基因Runx2、骨钙素、Ⅰ型胶原蛋白表达上调,碱性磷酸酶活性较高,证明JBMMSCs有良好的成骨分化能力。有学者通过实验比较JBMMSCs与牙周膜干细胞的成骨分化能力,分别将两组干细胞制成的细胞膜片植入骨缺损处,均可观察到大量的类骨质样新生骨形成,但JBMMSCs形成新骨的骨密度更高,证明JBMMSCs的成骨分化能力更强[14]。见图3。"
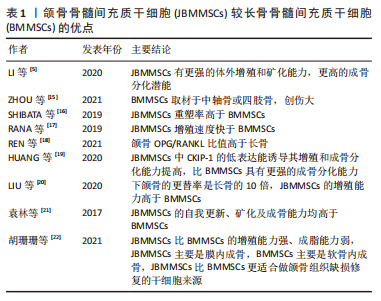
2.2 JBMMSCs较长骨来源骨髓间充质干细胞的优点 JBMMSCs可以从正颌手术及种植手术废弃的颌骨碎片、拔牙后的牙槽骨碎片中获得,具有来源丰富、不涉及二次创伤、取材方便等优点[5]。长骨来源骨髓间充质干细胞(bone marrow derived mesenchymal stem cells,BMMSCs)一般取材于中轴骨或四肢骨,相比JBMMSCs,取材费时且对供区造成的创伤较大[15]。在人的一生中颌骨都会受到咀嚼等活动产生的持续性机械刺激,有利于其不断重塑,重塑率高于长骨[16]。有研究表明用颌骨组织修复颌面部骨缺损比使用长骨的成骨效果更稳定,远期骨吸收更少,JBMMSCs增殖速度比从长骨中提取出的骨髓间充质干细胞更快,二者在成骨基因表达和细胞分化方面也有所不同[17]。REN等[18]在大鼠模型中发现JBMMSCs比骨髓间充质干细胞具有显著的促进破骨细胞分化的能力,颌骨中OPG/RANKL比值明显高于长骨,更利于调控正确的成骨细胞与破骨细胞之间的平衡。HUANG等[19]发现酪蛋白激酶2相互作用蛋白1 (casein kinase 2 interacting protein-1,CKIP-1)通过抑制Smurf1介导的Smad从而负向调节骨的形成,JBMMSCs中CKIP-1的低表达能诱导其增殖和成骨分化能力提高,与长骨骨髓间充质干细胞相比,JBMMSCs具有更强的成骨分化能力。LIU等[20]在犬模型中发现下颌骨的更替率是长骨的10倍,导致JBMMSCs的增殖能力高于骨髓间充质干细胞。袁林等[21]做了JBMMSCs和长骨骨髓间充质干细胞的比较研究,结果发现JBMMSCs比长骨骨髓间充质干细胞的克隆形成及增殖能力更强,成骨相关基因Ⅰ型胶原、Runx2、骨钙素的表达水平更高,且JBMMSCs的碱性磷酸酶活性、形成的矿化结节数量、早期成骨能力均明显高于长骨骨髓间充质干细胞,说明JBMMSCs的自我更新、矿化及成骨能力均高于长骨骨髓间充质干细胞。胡珊珊等[22]发现,无论是健康的小鼠还是绝经后骨质疏松状态的小鼠,JBMMSCs均比长骨骨髓间充质干细胞具有更强的增殖能力,且JBMMSCs比长骨骨髓间充质干细胞的成脂能力弱,JBMMSCs主要是以膜内成骨为主,而长骨骨髓间充质干细胞主要是软骨内成骨,说明JBMMSCs比长骨骨髓间充质干细胞更适合做颌骨组织缺损修复的干细胞来源。见表1。"
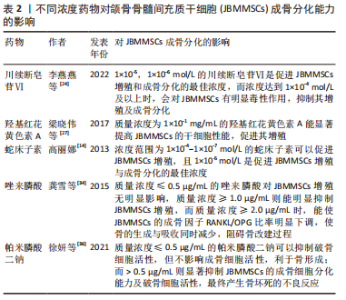
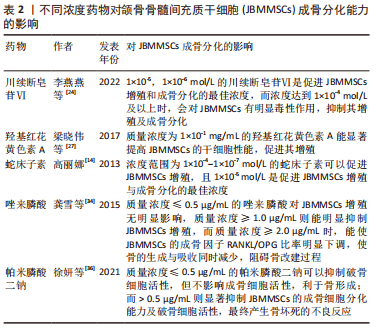
2.3 药物对JBMMSCs成骨分化的影响 2.3.1 中草药 (1)川续断皂苷Ⅵ( asperosaponin Ⅵ,ASA Ⅵ):续断是一种长期用于治疗骨疾病的中草药,川续断皂苷Ⅵ是从续断中提取出的生物活性三萜皂苷,具有抗炎、抗菌、促进成骨细胞分化及骨基质钙化的作用,有防止骨质疏松、促进骨伤愈合和保护神经的功效[23]。李燕燕等[24]发现低浓度的川续断皂苷Ⅵ对JBMMSCs增殖无毒性,并且能明显增强JBMMSCs的碱性磷酸酶活性,促进其成骨分化,且1×10-5,1×10-6 mol/L的川续断皂苷Ⅵ是促进JBMMSCs增殖和成骨分化的最佳浓度,而浓度达到1×10-4 mol/L及以上时,川续断皂苷Ⅵ会对JBMMSCs有明显毒性作用,抑制其增殖及成骨分化。但是目前文献未见川续断皂苷Ⅵ对JBMMSCs成骨分化作用机制的相关报道。 (2)羟基红花黄色素A(hydroxysafflor yellow A,HSYA):红花是一种传统中草药,属于菊科类植物,具有活血化瘀、通经止痛的作用,羟基红花黄色素A是从红花中提取出的活性成分,用于预防骨质疏松症、治疗心脑血管疾病和妇科疾病等[25]。有研究表明,对于卵巢切除后的大鼠骨质疏松模型,羟基红花黄色素A中的多酚化合物可以保护因雌激素缺乏引起的骨质丢失[26],且羟基红花黄色素A可以抑制或逆转JBMMSCs成脂分化,促进其成骨分化和增殖,利于新骨形成。梁晓伟等[27]通过检测NANOG、SOX2、OCT4的表达,发现羟基红花黄色素A的安全有效质量浓度为1×10-1 mg/mL,能显著提高JBMMSCs的干细胞性能,促进其增殖,成骨相关因子Runx2、骨钙素、Osterix、骨桥蛋白的表达上调,诱导并促进JBMMSCs的成骨分化,但高浓度羟基红花黄色素A反而对细胞增殖起抑制作用。因此适宜浓度的羟基红花黄色素A是促进JBMMSCs成骨分化的关键。 (3)蛇床子素(Osthole):蛇床子素是从传统中草药蛇床、欧前胡等药物中提取出的香豆素类化合物,具有抗炎、抗骨质疏松、抗细胞凋亡、促进细胞增殖和成骨分化的作用[28]。高丽娜[14]发现浓度范围为1×10-4-1×10-7 mol/L的蛇床子素可以促进JBMMSCs增殖,且1×10-5 mol/L是促进JBMMSCs增殖与成骨分化的最佳浓度,该浓度的蛇床子素能通过抑制RANKL和TRAP等基因及JNKI/2磷酸化水平而抑制骨吸收,并提高破骨细胞凋亡率;使JBMMSCs成骨相关基因Runx2、骨钙素及碱性磷酸酶活性明显提高,在加入1×10-5 mol/L浓度蛇床子素的JBMMSCs膜片中,Ⅰ型胶原、β1整合素、纤连蛋白的表达水平明显增高,可观察到细胞膜片在羟基磷灰石-磷酸三钙表面及支架材料间隙之间有大量的类骨质样新骨形成,并可见明显的成骨细胞及骨陷窝,有较好地促进JBMMSCs成骨分化的作用。 2.3.2 双膦酸盐(bisphosphonates,BPs) 主要治疗骨质疏松症、骨吸收、骨发育不良、恶性肿瘤晚期骨转移、Paget骨病等,但长期使用会诱发双膦酸盐类颌骨骨坏死[29]。成骨细胞是双膦酸盐的靶向细胞,双膦酸盐在成骨细胞的增殖、分化、合成细胞外基质蛋白及矿化过程中起调节作用,而双膦酸盐对破骨细胞则有直接的毒性作用,可使破骨细胞凋亡[30]。 (1)唑来膦酸(zoledronic acid):是双膦酸盐的第3代异环型含氮药物,是目前抑制骨吸收作用最强的双膦酸盐类药物[31]。有研究证实高浓度唑来膦酸会产生药物毒性作用,从而影响成骨细胞的碱性磷酸酶活性和矿化功能,抑制其增殖,但低浓度的唑来膦酸则不影响成骨细胞的增殖和分化,甚至可以促进细胞中矿化物的沉积[32]。国外学者发现唑来膦酸能够抑制核因子κB受体活化因子配体(receptor activator of NF-κB ligand,RANKL)诱导的破骨细胞分化和抗酒石酸酸性磷酸酶活性,打破成骨细胞和破骨细胞之间的平衡,导致骨形成和吸收同时减少,从而调节细胞的成骨分化[33]。龚雪等[34]通过建立大鼠实验模型,发现低质量浓度唑来膦酸(≤0.5 μg/mL)对JBMMSCs增殖无明显影响,质量浓度≥1.0 μg/mL则能明显抑制JBMMSCs增殖,而唑来膦酸质量浓度≥2.0 μg/mL时,能使JBMMSCs的RANKL mRNA表达降低,骨保护素mRNA的表达增加,成骨因子RANKL/OPG比率明显下调,不仅抑制破骨细胞的功能,还使骨的生成与吸收同时减少,阻碍骨改建过程,最终抑制JBMMSCs的增殖和成骨分化。 (2)帕米膦酸二钠(pamidronate disodium,PD):属于双膦酸盐类药物,能有效治疗骨吸收、骨发育不全与骨质疏松等疾病,帕米膦酸二钠可以吸附在破骨细胞表面使其凋亡,起抑制破骨细胞活性的作用[35]。徐妍等[36]发现≤0.5 μg/mL的帕米膦酸二钠可以负向调节成骨细胞到破骨细胞的诱导信号,抑制破骨细胞活性,但不影响成骨细胞活性,故利于骨形成;而帕米膦酸二钠质量浓度> 0.5 μg/mL则显著抑制JBMMSCs的成骨细胞分化能力及破骨细胞活性,最终产生骨坏死的不良反应;经茜素红S染色定量及qRT-PCR结果显示更低质量浓度的帕米膦酸二钠对JBMMSCs的成骨分化能力无明显影响。GAO等[37]发现帕米膦酸二钠能同时降低骨代谢与破骨细胞活性,使骨组织发生错误改建,抑制破骨细胞的前体细胞释放血小板来源生长因子,从而影响血管内皮生长因子及成骨分化因子的表达,抑制人颌骨内的血管生长,使颌骨骨组织血液供应不足,JBMMSCs无法进行正常的成骨分化,最终导致颌骨组织坏死的发生。?AHIN等[38]发现低浓度的帕米膦酸二钠不影响成骨细胞活性,但抑制破骨细胞活性,有利于促进骨的形成,而高浓度的帕米膦酸二钠不仅抑制破骨细胞内特定还原酶的活性,还抑制破骨细胞所需蛋白质的合成,使破骨细胞无法引导骨吸收,而影响骨的形成,最终使骨组织坏死。 多种药物可用于颌面部骨缺损修复的治疗,包括中草药、降钙素、双膦酸盐、地诺单抗和激素类药物。经查阅文献发现,部分中草药和双膦酸盐类药物已用于JBMMSCs成骨分化的研究,且药物的浓度对细胞成骨分化的影响至关重要。见表2。"
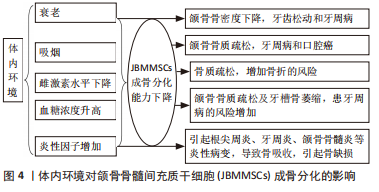
2.4 体内环境对JBMMSCs成骨分化的影响 2.4.1 衰老 衰老易引发骨质疏松,骨质疏松不仅表现在躯干骨和四肢骨,颌骨也会因骨质疏松而出现骨密度下降,引发牙齿松动和牙周病等疾病[39]。在正常生理状态下,成骨细胞和破骨细胞介导的骨形成与骨吸收保持平衡,以维持骨稳态,在骨质疏松发生后,骨稳态的平衡被打破,使骨的吸收大于骨的形成,造成骨质流失,骨密度下降[40]。李胜丹等[41]通过大鼠模型研究JBMMSCs成骨分化与年龄的关系,发现衰老组大鼠的颌骨和股骨均表现出骨质疏松,骨密度及骨小梁强度均下降,JBMMSCs成骨分化相关因子Runx2和骨钙素的表达明显低于年轻组,JBMMSCs的增殖和成骨分化随着年龄的增长而降低。SHEN等[42]发现雄性SD大鼠会随着衰老发展为骨质疏松症,间充质干细胞衰老、成骨分化能力下降而成脂能力增强,使生物学功能下降,最终导致骨衰老及退行性变的发生。DENG等[43]发现组蛋白去甲基化酶KDM4B在调节JBMMSCs成骨分化和颌骨老化中起重要作用,JBMMSCs中KDM4B缺失可打破骨脂肪平衡,抑制其成骨,但促进脂肪生成及衰老,因此KDM4B可能是颌骨衰老中决定其骨稳态的分子基础。南京医科大学附属口腔医院团队发现乙酰化酶Sirtuin1可以抗衰老[44],通过过表达去乙酰化酶蛋白Sirtuin 1可以增加Bmi1的去乙酰化和核转位,进而灭活p16信号通路,促进JBMMSCs增殖和成骨分化,抑制其衰老,预防老年性及雌激素缺乏引起的颌骨骨质疏松,故Sirtuin1可能成为年龄相关颌骨衰老的治疗靶点。 2.4.2 吸烟 吸烟是影响身体健康的危险因素之一,吸烟不仅可降低全身的骨密度,烟草中的尼古丁还可引起颌骨骨质疏松、牙周病和口腔癌等疾病[45]。KIM等[46]发现烟草中的尼古丁可调节成骨相关基因的表达,高浓度的尼古丁可抑制JBMMSCs的增殖和成骨分化能力,低浓度的尼古丁则可能起相反作用。赵喜聪[45]分别取吸烟和不吸烟患者的颌骨组织,分离培养获得JBMMSCs,对比研究吸烟对人JBMMSCs成骨分化的影响,发现吸烟组JBMMSCs的矿化结节面积、碱性磷酸酶活性、骨向分化相关基因Ⅰ型胶原、骨唾液酸蛋白、骨钙素的表达、新骨生成的数量、质量及细胞生长速度和体外增殖能力均明显低于非吸烟组,而非吸烟组干细胞的关键细胞受体STRO-1、CD146的表达量大于吸烟组,故吸烟降低了人JBMMSCs的成骨分化能力和增殖能力。还有动物实验表明,每天将一定剂量的尼古丁注射到大鼠皮下,可引起大鼠磨牙根分叉处的牙槽骨流失,引起牙松动,证明尼古丁加速了牙槽骨的吸收[47]。ROTHEM等[48]发现尼古丁能抑制JBMMSCs的骨唾液酸蛋白、骨钙素等成骨分化特异性蛋白合成,干扰矿物质沉积和矿物质支架的形成,降低细胞外基质的钙化程度和细胞碱性磷酸酶活性,而抑制骨的形成。 2.4.3 雌激素 雌激素水平对维持骨密度非常重要,在骨重塑过程中雌激素分泌减少会导致其骨吸收大于骨形成,使骨密度下降,易引起骨质疏松、骨折的风险增加[49]。有学者发现去除卵巢后的大鼠因雌激素水平降低,其JBMMSCs的Runx2及骨钙素在mRNA与蛋白水平上的表达及矿化结节形成能力均降低[50],说明雌激素水平降低对JBMMSCs的增殖与成骨能力产生负面影响。YU等[51]发现在低水平雌激素状态下,JBMMSCs的碱性磷酸酶活性升高,可能是骨质疏松状态下破骨细胞活性增高,从而使JBMMSCs的骨代谢水平升高以增加其代偿。LIU等[52]在不同时间点通过microCT对比观察切除卵巢后大鼠的颌骨与长骨骨量的变化,发现颌骨骨量出现显著下降的时间要晚于长骨,证明颌骨对雌激素水平降低引起的骨质流失敏感性比长骨低。DU等[53]发现尽管雌激素水平降低导致JBMMSCs增殖能力与碱性磷酸酶活性增高,但Runx2及骨钙素表达下调,最终降低了骨基质的沉积能力,使JBMMSCs成骨分化能力降低。故更年期妇女因体内雌激素水平降低,致使骨吸收大于骨形成,易出现绝经后骨质疏松,导致骨量减少,颌骨也会表现出相应的病变[54]。 2.4.4 血糖 糖尿病是一种全身性的代谢性疾病,患者常伴有钙、磷代谢异常和骨代谢紊乱,导致继发性骨质减少,使颌骨出现骨质疏松及牙槽骨萎缩等症状,患牙周疾病的风险增加[55]。GARCíA-HERNáNDEZ等[56]发现高糖环境下虽然干细胞的成骨能力增强,但矿化质量下降,钙/磷比值降低,最终影响细胞的成骨分化能力。陈杨等[57]发现葡萄糖浓度为5.5-25 mmol/L可以促进JBMMSCs的增殖,但浓度达44 mmol/L时,明显抑制细胞增殖;葡萄糖浓度在5.5-11 mmol/L范围内可促进JBMMSCs成骨分化,但随浓度增加,JBMMSCs的碱性磷酸酶活性、钙结节数量呈剂量依赖性下降,Runx2、骨钙素的表达均出现先上调再下调的趋势,最终抑制JBMMSCs成骨分化,使骨的形成减少,骨密度降低。YOU等[58]发现一定范围内的高糖环境能为细胞生长提供能量,若浓度过高,细胞因无法抵抗高糖产生的高渗毒性,反而抑制JBMMSCs生长和增殖,并呈剂量依赖性,使成骨分化能力下降。因此,体内血糖浓度在正常范围内可以促进JBMMSCs增殖和成骨分化,而高血糖则起抑制作用,使细胞成骨分化能力下降,骨密度降低,引起相应的骨疾病。 2.4.5 炎性因子 炎性因子会引起颌面部相应的炎性病变,如根尖周炎、牙周炎、颌骨骨髓炎,颌面部损伤也常伴炎症状态,不仅会导致细胞成骨能力下降,还会导致骨吸收而引起骨缺损的发生[59]。白细胞介素6、白细胞介素1β及肿瘤坏死因子α是常见炎性因子[60]。王璞等[61]用20 ng/mL的肿瘤坏死因子α作用于JBMMSCs 3 d,发现细胞几乎不发生凋亡,且在3 d的短时间使用质量浓度为100 ng/mL的肿瘤坏死因子α可作为不使JBMMSCs凋亡的最大浓度;而肿瘤坏死因子α在20 ng/mL质量浓度下作用于JBMMSCs 7 d后,因使细胞长期处于炎性微环境中,则导致了细胞的凋亡,故肿瘤坏死因子α作用于细胞的浓度及时间均影响JBMMSCs的成骨分化。有研究发现糖原合成酶激酶3β为NF-κB通路的中心因子,激活糖原合成酶激酶3β可导致白细胞介素6、白细胞介素1β及肿瘤坏死因子α等多种炎性因子释放,而敲减糖原合成酶激酶3β能使β-catenin在胞浆内累积和入核,进而激活Wnt通路,使JBMMSCs成骨分化能力明显增强,因此抑制糖原合成酶激酶3β能促进JBMMSCs的成骨分化,减少炎症环境对其成骨的负面影响,为改善炎症条件下JBMMSCs骨再生提供思路[60]。见图4。"
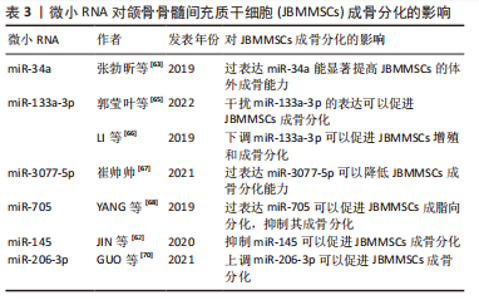
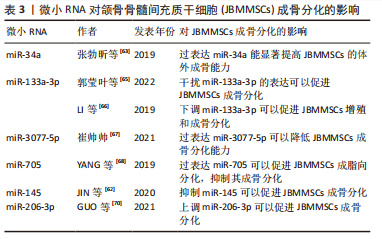
2.5 微小RNA(microRNA,miRNA)对JBMMSCs成骨分化的影响 miRNA是一种短链内源性小分子非编码RNA,可通过调节靶基因的表达而达到调控细胞增殖、分化和凋亡的目的,多种miRNA可以调控干细胞的成骨分化[62]。 2.5.1 miR-34a 张勃昕等[63]发现在诱导JBMMSCs成骨分化过程中,miR-34a家族的miRNA表达量均上调,其中以miR-34a升高最明显,且过表达miR-34a能显著提高JBMMSCs的体外成骨能力。因为miR-34a可以调控DKK1分泌蛋白,DKK1与WNT配体竞争性结合低密度脂蛋白受体相关蛋白,而抑制成骨分化经典信号通路Wnt/β-Catenin,且miR-34a可以通过抑制DKK1而激活Wnt/β-catenin信号通路,促进JBMMSCs成骨分化,使其成骨分化相关基因表达上调[63]。还有研究证明在JBMMSCs中过表达miR-34a可以抑制DKK1蛋白表达,而敲减miR-34a 能促进DKK1蛋白表达,从而调控JBMMSCs成骨分化[64]。 2.5.2 miR-133a-3p 郭莹叶等[65]发现在诱导JBMMSCs成骨分化过程中,骨形态发生蛋白9的表达显著升高,而miR-133a-3p可靶向调控骨形态发生蛋白9使其水平降低,从而激活Smad信号通路,抑制JBMMSCs的增殖和成骨分化,故干扰miR-133a-3p的表达可以显著提高JBMMSCs的活力。LI等[66]发现下调miR-133a-3p可以促进JBMMSCs增殖和成骨分化相关标志物Runx2、骨钙素和骨桥蛋白的表达,促进JBMMSCs成骨分化,抑制细胞凋亡。 2.5.3 miR-3077-5p 崔帅帅[67]通过绝经后骨质疏松小鼠模型发现miR-3077-5p在成骨诱导过程中的表达量随时间逐渐降低,在成脂诱导过程中的表达量随时间逐渐升高,过表达miR-3077-5p可以打破JBMMSCs成骨分化和成脂分化之间的平衡,降低其成骨分化能力,增强成脂分化能力。 2.5.4 miR-705 YANG等[68]通过建立骨质疏松小鼠实验模型,发现在JBMMSCs中过表达miR-705可以促进JBMMSCs成脂向分化,抑制其成骨分化。miR-705不仅在绝经后的长骨骨质疏松中有一定的调节作用,而且对JBMMSCs的成骨分化能力有抑制作用[69]。 2.5.5 miR-145 JIN等[62]通过实验发现miR-145可以直接靶向调控SEMA3A,并表明miR-145作为抑制因子,在JBMMSCs成骨分化中起重要作用,因miR-145在JBMMSCs成骨分化过程中下调,可以靶向抑制成骨的正调节剂SEMA3A,故抑制miR-145可以使碱性磷酸酶活性增加、矿化程度以及成骨分化标志物Runx2、Osterix和Ⅰ型胶原的表达水平增加,促进JBMMSCs成骨分化。 2.5.6 miR-206-3p Guo等[70]发现miR-206-3p是GATA结合蛋白4的重要下游因子,GATA结合蛋白4在颌骨发育中起重要作用,如果缺乏则导致颌骨发育缺陷;miR-206-3p通过靶向激活T细胞的骨形态发生蛋白3和核因子细胞质1从而在颌骨发育中发挥关键作用,实验证明局部敲低miR-206-3p可使JBMMSCs成骨分化相关基因的表达水平降低,RANKL的蛋白质水平和 RANKL/OPG比率提高,抑制骨形成,促进骨吸收,而上调miR-206-3p可以增加成骨标志物的水平,增强骨小梁结构和减少破骨细胞数量,促进JBMMSCs成骨分化。见表3。"
| [1] KLÜTER T, HASSAN R, RASCH A, et al. An Ex Vivo Bone Defect Model to Evaluate Bone Substitutes and Associated Bone Regeneration Processes. Tissue Eng Part C Methods. 2020;26(1):56-65. [2] AYUB N, FARAJ M, GHATAN S, et al. The Treatment Gap in Osteoporosis. J Clin Med. 2021;10(13):3002. [3] PENG J, CHEN L, PENG K, et al. Bone Marrow Mesenchymal Stem Cells and Endothelial Progenitor Cells Co-Culture Enhances Large Segment Bone Defect Repair. J Biomed Nanotechnol. 2019;15(4):742-755. [4] KIM HK, LEE SG, LEE SW, et al. A Subset of Paracrine Factors as Efficient Biomarkers for Predicting Vascular Regenerative Efficacy of Mesenchymal Stromal/Stem Cells. Stem Cells. 2019;37(1):77-88. [5] LI C, WANG F, ZHANG R, et al. Comparison of Proliferation and Osteogenic Differentiation Potential of Rat Mandibular and Femoral Bone Marrow Mesenchymal Stem Cells In Vitro. Stem Cells Dev. 2020;29(11):728-736. [6] ZHANG W, DONG Z, LI D, et al. Cathepsin K deficiency promotes alveolar bone regeneration by promoting jaw bone marrow mesenchymal stem cells proliferation and differentiation via glycolysis pathway. Cell Prolif. 2021;54(7):e13058. [7] LI L, LI J, ZOU Q, et al. Enhanced bone tissue regeneration of a biomimetic cellular scaffold with co-cultured MSCs-derived osteogenic and angiogenic cells. Cell Prolif. 2019 ;52(5):e12658. [8] LAI K, XI Y, DU X, et al. Activation of Nell-1 in BMSC Sheet Promotes Implant Osseointegration Through Regulating Runx2/Osterix Axis. Front Cell Dev Biol. 2020;8(9):868. [9] 程兵坤,梁建飞,秦东泽,等.高纯度大鼠下颌骨来源间充质干细胞的分离、培养及生物学特性[J]. 中国组织工程研究,2021,25(1):67-72. [10] REDONDO LM, GARCÍA V, PERAL B, et al. Repair of maxillary cystic bone defects with mesenchymal stem cells seeded on a cross-linked serum scaffold. J Craniomaxillofac Surg. 2018;46(2):222-229. [11] CHOI YH, HAN Y, JIN SW, et al. Pseudoshikonin I enhances osteoblast differentiation by stimulating Runx2 and Osterix. J Cell Biochem. 2018; 119(1):748-757. [12] LUO J, XU J, CAI J, et al. The In Vitro and In Vivo Osteogenic Capability of the Extraction Socket-Derived Early Healing Tissue. J Periodontol. 2016; 87(9):1057-1066. [13] NAKAJIMA R, ONO M, HARA ES, et al. Mesenchymal stem/progenitor cell isolation from tooth extraction sockets. J Dent Res. 2014;93(11): 1133-1140. [14] 高丽娜. 蛇床子素对人牙周膜干细胞和颌骨骨髓间充质干细胞膜片形成和生物学性能的影响[D].西安:第四军医大学,2013. [15] ZHOU B, PENG K, WANG G, et al. Polo Like Kinase 4 (PLK4) impairs human bone marrow mesenchymal stem cell (BMSC) viability and osteogenic differentiation. Biochem Biophys Res Commun. 2021; 549(4):221-228. [16] SHIBATA S, TAKAHASHI M, FUJIKAWA K. Histochemical and Ultrastructural Study of Developing Gonial Bone With Reference to Initial Ossification of the Malleus and Reduction of Meckel’s Cartilage in Mice. Anat Rec (Hoboken). 2019;302(11):1916-1933. [17] RANA D, KUMAR S, WEBSTER TJ, et al. Impact of Induced Pluripotent Stem Cells in Bone Repair and Regeneration. Curr Osteoporos Rep. 2019; 17(4):226-234. [18] REN S, JIAO G, ZHANG L, et al. Bionic Tiger-Bone Powder Improves Bone Microstructure and Bone Biomechanical Strength of Ovariectomized Rats. Orthop Surg. 2021;13(3):1111-1118. [19] HUANG X, CHENG B, SONG W, et al. Superior CKIP-1 sensitivity of orofacial bone-derived mesenchymal stem cells in proliferation and osteogenic differentiation compared to long bone-derived mesenchymal stem cells. Mol Med Rep. 2020;22(2):1169-1178. [20] LIU LN, ZHANG XH, LIU HH, et al. Osteogenesis Differences Around Titanium Implant and in Bone Defect Between Jaw Bones and Long Bones. J Craniofac Surg. 2020;31(8):2193-2198. [21] 袁林,钱钧,杨征毅,等.不同来源骨髓间充质干细胞成骨能力的比较[J].口腔疾病防治,2017,25(9):554-559. [22] 胡珊珊,张为,曹炜,等.雌性健康和绝经小鼠长骨及颌骨来源骨髓间充质干细胞生物学特性的比较[J].贵州医科大学学报,2021,46(12): 1389-1395. [23] 赵金龙,梁桂洪,韩燕鸿,等.川续断提取物续断皂苷Ⅵ防治骨质疏松症的研究进展[J].中国骨质疏松杂志,2020,26(5):755-759. [24] 李燕燕,朱珠,谢雯静,等.川续断皂苷Ⅵ对人颌骨骨髓间充质干细胞成骨分化的影响[J].口腔医学,2022,42(3):204-209. [25] LI CY, YIN JG, ZHANG J, et al. Pharmacokinetic profiles of hydroxysafflor yellow A following intravenous administration of its pure preparations in healthy Chinese volunteers. J Ethnopharmacol. 2015;162(3):225-230. [26] ZHOU MX, FU JH, ZHANG Q, et al. Effect of hydroxy safflower yellow A on myocardial apoptosis after acute myocardial infarction in rats. Genet Mol Res. 2015;14(2):3133-3141. [27] 梁晓伟,李阳飞,李琥,等.羟基红花黄色素A对人骨髓间充质干细胞成骨分化的影响[J].口腔生物医学,2017,8(2):90-94. [28] MO Y, WU Y, LI X, et al. Osthole delays hepatocarcinogenesis in mice by suppressing AKT/FASN axis and ERK phosphorylation. Eur J Pharmacol. 2020;867(1):172788. [29] DONG X, HE L, ZANG X, et al. Adipose-Derived Stem Cells Promote Bone Coupling in Bisphosphonate-Related Osteonecrosis of the Jaw by TGF-β1. Front Cell Dev Biol. 2021;9(5):639590. [30] KIM DS, KIM JH, OHE JY, et al. Bisphosphonate-related osteonecrosis of the jaw in a patient with osteoporosis following treatment of testicular cancer: a case report. J Korean Assoc Oral Maxillofac Surg. 2015;41(6):327-331. [31] RODRÍGUEZ-LOZANO FJ, OÑATE-SÁNCHEZ R, GONZÁLVEZ-GARCÍA M, et al. Allogeneic Bone Marrow Mesenchymal Stem Cell Transplantation in Tooth Extractions Sites Ameliorates the Incidence of Osteonecrotic Jaw-Like Lesions in Zoledronic Acid-Treated Rats. J Clin Med. 2020;9(6):1649. [32] PAN B, TO LB, FARRUGIA AN, et al. The nitrogen-containing bisphosphonate, zoledronic acid, increases mineralisation of human bone-derived cells in vitro. Bone. 2004;34(1):112-123. [33] YANG G, KIM YN, KIM H, et al. Effect of Human Umbilical Cord Matrix-Derived Mesenchymal Stem Cells on Bisphosphonate-Related Osteonecrosis of the Jaw. Tissue Eng Regen Med. 2021;18(6):975-988. [34] 龚雪,苏俭生.唑来膦酸对大鼠颌骨间充质干细胞成骨分化的影响[J].口腔颌面外科杂志,2015,25(1):28-33. [35] ARGENTIERO A, SOLIMANDO AG, BRUNETTI O, et al. Skeletal Metastases of Unknown Primary: Biological Landscape and Clinical Overview. Cancers (Basel). 2019;11(9):1270. [36] 徐妍,孙晋,周海华.帕米膦酸二钠对颌骨来源间充质干细胞成骨分化的影响[J].中国口腔种植学杂志,2021,26(4):219-225. [37] GAO SY, LIN RB, HUANG SH, et al. PDGF-BB exhibited therapeutic effects on rat model of bisphosphonate-related osteonecrosis of the jaw by enhancing angiogenesis and osteogenesis. Bone. 2021;144(3):115117. [38] ŞAHIN O, ODABAŞI O, ALIYEV T, et al. Risk factors of medication-related osteonecrosis of the jaw: a retrospective study in a Turkish subpopulation. J Korean Assoc Oral Maxillofac Surg. 2019;45(2):108-115. [39] GUERVILLE F, DE SOUTO BARRETO P, ADER I, et al. Revisiting the Hallmarks of Aging to Identify Markers of Biological Age. J Prev Alzheimers Dis. 2020; 7(1):56-64. [40] DI MICCO R, KRIZHANOVSKY V, BAKER D, et al. Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat Rev Mol Cell Biol. 2021;22(2):75-95. [41] 李胜丹,梁乙然,刘一涵.衰老性骨质疏松微环境对颌骨骨髓间充质干细胞生物学功能的影响[J]. 中华老年口腔医学杂志,2019,17(4):198-203,238. [42] SHEN X, SI Y, FU Y, et al. MicroRNA-31a-5p from aging BMSCs links bone formation and resorption in the aged bone marrow microenvironment. Aging Cell. 2018;17(4):e12794. [43] DENG P, CHANG I, WANG J, et al. Loss of KDM4B impairs osteogenic differentiation of OMSCs and promotes oral bone aging. Int J Oral Sci. 2022;14(1):24. [44] 南京医科大学附属口腔医院. Sirtuin 1经Bmi1介导调控老年性牙槽骨丢失作用机制的研究方法:CN201810435680.8[P]. 2020-12-29. [45] 赵喜聪.吸烟者人颌骨骨髓间充质干细胞增殖和骨向分化能力的对比研究[D].西安:第四军医大学,2012. [46] KIM BS, KIM SJ, KIM HJ, et al. Effects of nicotine on proliferation and osteoblast differentiation in human alveolar bone marrow-derived mesenchymal stem cells. Life Sci. 2012;90(3-4):109-115. [47] BOSCO AF, BONFANTE S, DE ALMEIDA JM, et al. A histologic and histometric assessment of the influence of nicotine on alveolar bone loss in rats. J Periodontol. 2007;78(3):527-532. [48] ROTHEM DE, ROTHEM L, SOUDRY M, et al. Nicotine modulates bone metabolism-associated gene expression in osteoblast cells. J Bone Miner Metab. 2009;27(5):555-561. [49] PAGANI S, FINI M, GIAVARESI G, et al. The active role of osteoporosis in the interaction between osteoblasts and bone metastases. Bone. 2015;79: 176-182. [50] 许雄程,阳雪,何梦娇,等.去卵巢对大鼠颌骨成骨细胞增殖与成骨分化能力的影响[J].中国骨质疏松杂志,2018,24(5):567-572. [51] YU SJ, LIU HC, LING-LING E, et al. Proliferation and differentiation of osteoblasts from the mandible of osteoporotic rats. Exp Biol Med (Maywood). 2012;237(4):395-406. [52] LIU XL, LI CL, LU WW, et al. Skeletal site-specific response to ovariectomy in a rat model: change in bone density and microarchitecture. Clin Oral Implants Res. 2015;26(4):392-398. [53] DU Z, LEE RS, HAMLET S, et al. Evaluation of the first maxillary molar post-extraction socket as a model for dental implant osseointegration research. Clin Oral Implants Res. 2016;27(12):1469-1478. [54] ABRAHAM A, COHEN A, SHANE E. Premenopausal bone health: osteoporosis in premenopausal women. Clin Obstet Gynecol. 2013;56(4):722-729. [55] PRESSMAN AR, KINOSHITA L, KIRK S, et al. A novel telemonitoring device for improving diabetes control: protocol and results from a randomized clinical trial. Telemed J E Health. 2014;20(2):109-114. [56] GARCÍA-HERNÁNDEZ A, ARZATE H, GIL-CHAVARRÍA I, et al. High glucose concentrations alter the biomineralization process in human osteoblastic cells. Bone. 2012;50(1):276-288. [57] 陈杨,胡赟,杨兰,等.不同糖浓度对颌骨骨髓间充质干细胞成骨分化的影响[J].四川大学学报(医学版),2016,47(5):679-684. [58] YOU L, GU W, CHEN L, et al. MiR-378 overexpression attenuates high glucose-suppressed osteogenic differentiation through targeting CASP3 and activating PI3K/Akt signaling pathway. Int J Clin Exp Pathol. 2014;7(10): 7249-7261. [59] KATAGIRI W, TAKEUCHI R, SAITO N, et al. Migration and phenotype switching of macrophages at early-phase of bone-formation by secretomes from bone marrow derived mesenchymal stem cells using rat calvaria bone defect model. J Dent Sci. 2022;17(1):421-429. [60] 胡祥翔,胡开进,赵铱民.炎性微环境中糖原合成酶激酶-3β对颌骨来源骨髓间充质干细胞成骨分化影响研究[J].中国实用口腔科杂志, 2017,10(1):40-43. [61] 王璞,韦丽宾,倪广晓,等. TNF-α对颌骨骨髓间充质与牙周膜两种干细胞自噬水平的影响[J]. 现代口腔医学杂志,2020,34(1):14-16. [62] JIN Y, HONG F, BAO Q, et al. MicroRNA-145 suppresses osteogenic differentiation of human jaw bone marrow mesenchymal stem cells partially via targeting semaphorin 3A. Connect Tissue Res. 2020;61(6): 577-585. [63] 张勃昕,吉爱红,曹正垚,等. miR-34a对人颌骨骨髓间充质干细胞成骨分化的影响[J].口腔生物医学,2019,10(1):1-5. [64] XIAO T, FU Y, ZHU W, et al. HDAC8, A Potential Therapeutic Target, Regulates Proliferation and Differentiation of Bone Marrow Stromal Cells in Fibrous Dysplasia. Stem Cells Transl Med. 2019;8(2):148-161. [65] 郭莹叶,高建华,郭永梅,等. miR-133a-3p靶向BMP9调控人颌骨骨髓间充质干细胞增殖、分化和凋亡的研究[J].实用口腔医学杂志,2022, 38(2):253-258. [66] LI Q, XING W, GONG X, et al. RETRACTED: Astragalus polysaccharide promotes proliferation and osteogenic differentiation of bone mesenchymal stem cells by down-regulation of microRNA-152. Biomed Pharmacother. 2019;115(7):108927. [67] 崔帅帅. miRNA-3077-5p对绝经后骨质疏松小鼠颌骨BMSCs分化的影响[D].遵义:遵义医科大学,2021. [68] YANG XH, YANG K, AN YL, et al. MicroRNA-705 regulates the differentiation of mouse mandible bone marrow mesenchymal stem cells. PeerJ. 2019;7: e6279. [69] LIAO L, YANG X, SU X, et al. Redundant miR-3077-5p and miR-705 mediate the shift of mesenchymal stem cell lineage commitment to adipocyte in osteoporosis bone marrow. Cell Death Dis. 2013;4(4):e600. [70] GUO S, GU J, MA J, et al. GATA4-driven miR-206-3p signatures control orofacial bone development by regulating osteogenic and osteoclastic activity. Theranostics. 2021;11(17):8379-8395. |
| [1] | Tang Haotian, Liao Rongdong, Tian Jing. Application and design of piezoelectric materials for bone defect repair [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 1117-1125. |
| [2] | Qin Yuxing, Ren Qiangui, Li Zilong, Quan Jiaxing, Shen Peifeng, Sun Tao, Wang Haoyu. Action mechanism and prospect of bone microvascular endothelial cells for treating femoral head necrosis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 955-961. |
| [3] | Zhang Min, Zhang Xiaoming, Liu Tongbin. Application potential of naringin in bone tissue regeneration [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(5): 787-792. |
| [4] | Xiong Wei, Yuan Lingmei, Qian Guowen, Huang Jinyang, Pan Bin, Guo Ling, Zeng Zhikui. Value of a critical bone defect animal model in evaluating osteogenic efficacy of bone tissue engineering scaffold [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(35): 5714-5720. |
| [5] | Liu Zixuan, Li Yan, Ji Lin, Xia Delin. Biological properties of nano-hydroxyapatite-zinc oxide composite scaffolds and their effects on the behavior of MC3T3-E1 osteoblasts [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(34): 5441-5447. |
| [6] | You Yan, Chen Jiawen, Lin Binbin, Wu Jingyi, Liu Peng, Wu Buling, Sun Tianyu. Mechanism and application of glycosaminoglycan in bone tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(34): 5538-5545. |
| [7] | Xu Zhengyi, Wan Qianbing, Chen Junyu. Natural small molecular compounds in the treatment of bone-related diseases by regulating type H blood vessels and its application in tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(34): 5546-5553. |
| [8] | Wang Xinjie, Wang Guodong, Zheng Zhongren, Shao Yiming, Wang Jialiang, Ma Hui, Zhao Xiaowei. Manganese-containing bioceramic materials in the field of bone repair [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(34): 5570-5576. |
| [9] | Xu Rongwei, Wang Hao, Fu Qiuyue, Lan Xingming, Yang Kun. Bidirectional interaction between inflammatory factors and dental pulp stem cells during bone regeneration [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(33): 5385-5393. |
| [10] | Zhang Qingmei, Zhang Lupeng, Du Xiujuan, Li Bing. Application of carbon dots-based materials in bone tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(30): 4883-4889. |
| [11] | Zhang Xiaoyu, Chen Qi, Yang Xing, Hao Yuefeng. Application of poly(lactic-co-glycolic acid) copolymer microspheres in bone tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(30): 4896-4903. |
| [12] | Yu Aimin, Xu Ting, Zhu Yunying, Liang Jianqiang, Wu Donghui. Physicochemical properties of chitosan/bone powder/cellulose nanocrystals scaffold loaded with antimicrobial peptides for jaw repair [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(25): 3999-4005. |
| [13] | Long Zhisheng, Fu Liuxiang, Gong Feipeng, Wen Jiabin, Deng Ying, Min Huan, Deng Zhuan, Chen Gang. Expression and significance of pyroptosis associated protein in peripheral tissues with tantalum cage loosening [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(25): 4057-4062. |
| [14] | Qi Junqiang, Guo Chao, Niu Dongyang, Wang Haotian, Xiao Bing, Xu Guohua. Characteristics and application of bone repair materials of metal ion doped hydroxyapatite [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(21): 3415-3422. |
| [15] | Hou Jianming, Li Qi. Bioactive scaffolds in repairing osteoporotic bone defects [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(21): 3423-3429. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||