中国组织工程研究 ›› 2026, Vol. 30 ›› Issue (17): 4417-4429.doi: 10.12307/2026.104
• 组织构建综述 tissue construction review • 上一篇 下一篇
体外冲击波抗组织纤维化的应用及分子机制
黄思璟1,崔 瑞2,耿珑玉1,高蓓瑶2,葛瑞东2,江 山2
- 1北京体育大学运动医学与康复学院,北京市 100084;2中日友好医院康复医学科,北京市 100029
-
收稿日期:2025-03-21接受日期:2025-06-26出版日期:2026-06-18发布日期:2025-12-02 -
通讯作者:葛瑞东,博士,副主任治疗师,中日友好医院康复医学科,北京市 100029 并列通讯作者:江山,主任医师,副教授,中日友好医院康复医学科,北京市 100029 -
作者简介:黄思璟,女,2001年生,福建省南平市人,汉族,北京体育大学在读硕士,主要从事冲击波生物学效应方面的研究。 -
基金资助:中央高水平医院临床科研业务费资助课题(2023-NHLHCRF-YYPPLC-TJ-19),项目负责人:葛瑞东;中央高水平医院临床科研业务费资助课题(2023-NHLHCRF-YSPY-01),项目负责人:江山;国家重点研发计划项目(2022YFC2009700,2022YFC2009706),子课题负责人:江山
Application and molecular mechanism of extracorporeal shock wave for anti-fibrosis
Huang Sijing1, Cui Rui2, Geng Longyu1, Gao Beiyao2, Ge Ruidong2, Jiang Shan2
- 1School of Sports Medicine and Rehabilitation, Beijing Sport University, Beijing 100084, China; 2Department of Rehabilitation Medicine, China-Japan Friendship Hospital, Beijing 100029, China
-
Received:2025-03-21Accepted:2025-06-26Online:2026-06-18Published:2025-12-02 -
Contact:Ge Ruidong, PhD, Associate chief therapist, Department of Rehabilitation Medicine, China-Japan Friendship Hospital, Beijing 100029, China Co-corresponding author: Jiang Shan, Chief physician, Associate professor, Department of Rehabilitation Medicine, China-Japan Friendship Hospital, Beijing 100029, China -
About author:Huang Sijing, MS candidate, School of Sports Medicine and Rehabilitation, Beijing Sport University, Beijing 100084, China -
Supported by:the National High-Level Hospital Clinical Research Funding, Nos. 2023-NHLHCRF-YYPPLC-TJ-19 (to GRD) and 2023-NHLHCRF-YSPY-01 (to JS); National Key Research and Development Program of China, Nos. 2022YFC2009700 and 2022YFC2009706 (both to JS)
摘要:
文题释义:
体外冲击波:是一种高强度、短时持续的机械波,通常由专门的设备产生并通过皮肤传递至目标部位,产生的机械刺激可对生物组织产生一系列的生物物理效应。
纤维化:是组织对持续性损伤、炎症或代谢异常的一种修复反应。当修复过度或失调时,过多的纤维结缔组织会取代正常的功能性组织,损害组织的正常结构及功能。
背景:现有研究发现,体外冲击波可有效抑制组织纤维化形成,但有关体外冲击波抗纤维化的实验研究结果与总结较少,且未对相关信号通路进行归纳整理。
目的:综述目前临床或临床前原始研究,对体外冲击波在纤维化组织中的应用及相关分子机制进行归纳总结,为临床治疗纤维化疾病提供新思路。
方法:使用计算机检索PubMed、Web of Science以及中国知网、万方、维普数据库中的相关原创性研究,检索时限为2014年1月至2024年9月,英文检索词为“extracorporeal shockwave therapy,shock wave therapy,shock wave,fibrosis,fibroses”等,中文检索词为“冲击波,体外冲击波疗法,纤维化,抗纤维化”,采用主题词和自由词结合的方式进行检索。依据纳入排除标准对检索结果进行筛选、排除,最终纳入67篇文献进行综述分析。
结果与结论:①体外冲击波对多种组织纤维化相关疾病有益,可有效降低组织纤维化程度,改善患者临床症状;②体外冲击波主要通过转化生长因子β1、丝裂原活化蛋白激酶、血管生长因子以及炎症信号转导通路,影响纤维化相关细胞因子表达及细胞外基质组成,从而抑制纤维化过度形成;③目前纳入的临床研究数量较少,缺少临床数据支持;另外由于各研究的干预对象及体外冲击波干预方案差异大,实验结果单一,可能对体外冲击波抗纤维化的具体量效机制及总体分子作用体系产生影响;④研究结果表明,体外冲击波未来或可作为一种有效治疗手段参与到纤维化相关疾病的临床治疗中。
https://orcid.org/0009-0003-4506-5473(黄思璟); https://orcid.org/0000-0002-6082-8032(葛瑞东)
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
中图分类号:
引用本文
黄思璟, 崔 瑞, 耿珑玉, 高蓓瑶, 葛瑞东, 江 山. 体外冲击波抗组织纤维化的应用及分子机制[J]. 中国组织工程研究, 2026, 30(17): 4417-4429.
Huang Sijing, Cui Rui, Geng Longyu, Gao Beiyao, Ge Ruidong, Jiang Shan. Application and molecular mechanism of extracorporeal shock wave for anti-fibrosis[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(17): 4417-4429.
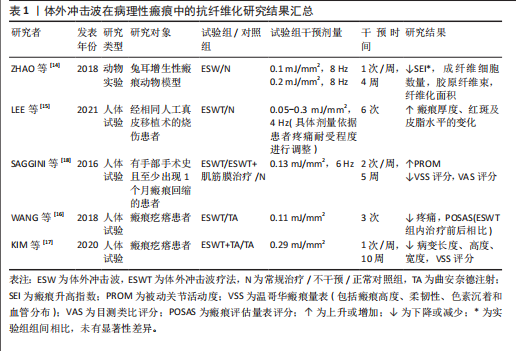
2.1.1 病理性瘢痕 皮肤瘢痕通常通过伤口愈合的方式发展,网状真皮和皮下组织的几种损伤(烧伤、手术、擦伤和撕裂等)均可能导致皮肤瘢痕形成。其中,病理性瘢痕可分为增生性瘢痕和回缩性瘢痕两种,前者又可分为单纯增生性瘢痕和瘢痕疙瘩[13]。
增生性瘢痕组织通常表现为成纤维细胞显著增加、纤维连接蛋白过度表达及平行于表皮排列的波状胶原束[14]。ZHAO等[14]在兔耳增生性瘢痕模型中发现不同能流密度的体外冲击波干预均可显著降低瘢痕增生指数,且连续干预35 d后相关组织的成纤维细胞数量、纤维化面积显著减少。同样,一项临床研究结果表明体外冲击波疗法较标准治疗显著改善了烧伤后增生性瘢痕患者的瘢痕厚度、红斑变化及皮脂水分[15]。瘢痕疙瘩与增生性瘢痕相比通常表现为延迟发作,在数月内出现旺盛的、不确定的胶原蛋白生长,形成坚固的宽结节[16],于病灶内注射曲安奈德是临床常见的治疗方法[7]。WANG等[16]经临床试验证实体外冲击波疗法较注射曲安奈德更能有效改善瘢痕外观,且体外冲击波疗法组治疗后瘢痕疼痛及瘢痕评估量表评分均较治疗前显著降低。KIM等[17]发现接受曲安奈德注射和体外冲击波疗法联合治疗患者较仅接受曲安奈德注射治疗患者的相关病变长度、宽度和高度显著下降,温哥华瘢痕量表评分显著降低,且更少出现不良反应。回缩性瘢痕的特征是胶原纤维呈索状排列,对周围健康组织施加显著的牵引力。SAGGINI等[18]研究发现体外冲击波疗法可显著降低患有手部回缩瘢痕患者的温哥华瘢痕量表评分及疼痛目测类比评分,提高手部的被动关节活动度。
如表1 所示,近年来体外冲击波在病理性瘢痕治疗中的抗纤维化作用已受到广泛关注。上述多项研究结果均已证实,不论是在动物实验或是人体试验中,体外冲击波均对病理性瘢痕表现出有益作用。相较于传统治疗手段(如局部类固醇注射、激光消融),体外冲击波可通过非侵入性机械刺激,有效降低瘢痕组织的纤维化密度,改善瘢痕瘙痒、疼痛及功能受限等临床症状,且未观察到不良反应产生。但目前在此方面的研究大多集中于临床疗效的观察,缺乏体外冲击波抗皮肤组织纤维化的分子机制研究,还需日后开展更多基础实验研究加以验证。
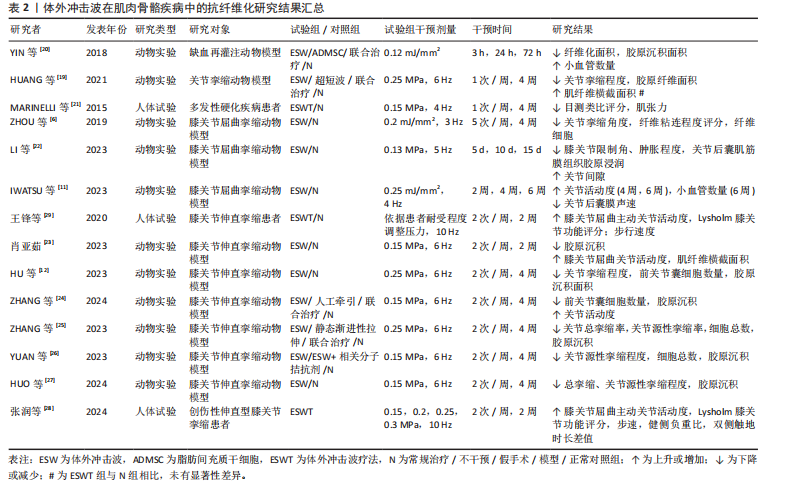
2.1.2 肌肉骨骼疾病 长时间、不适当的制动以及缺乏有效的机械刺激等是导致骨骼肌纤维化和关节挛缩出现的常见原因。其中,骨骼肌纤维化的病理特征表现为肌内膜和肌周结缔组织过度堆积,肌肉延展性下降,胶原
纤维逐渐取代正常肌肉纤维堆积于组织中[19]。一项研究结果表明,肌肉细胞在缺血情况下将极易出现纤维化改变,而体外冲击波干预能有效降低股四头肌缺血再灌注动物模型肌肉缺血区域的纤维化和胶原沉积面积,影响组织内纤维化及抗纤维化相关蛋白表达[20]。同时,HUANG等[19]在早期关节挛缩动物模型中发现,体外冲击波的机械刺激作用可抑制实验兔组织中缺氧诱导因子1α和转化生长因子β1的表达,同时其空化作用加速了血液微循环,从而有效降低肌源性挛缩水平及胶原纤维面积。另外,在一项针对多发性硬化患者的临床试验中,体外冲击波疗法对脊髓兴奋性缺乏影响,却显著降低了患者小腿三头肌的非反射性肌张力。因此,研究者们推测体外冲击波疗法是通过减少肌肉纤维化程度而实现相应疗效的[21]。
除长期关节制动外,关节挛缩可能由创伤、肌肉无力和神经功能障碍等多种原因导致[11],其一般由炎症反应引发,随后纤维组织广泛生长,粘连组织成熟为瘢痕带[6],抑制相应关节活动度,严重者或将造成关节畸形,引发肢体失用。多项研究结果显示,体外冲击波可有效改善关节屈曲、伸直挛缩动物模型关节活动度,降低胶原沉积面积,增大关节间隙及相关肌纤维横截面积;同时在分子层面上,体外冲击波可降低Ⅰ型胶原、α-平滑肌肌动蛋白及转化生长因子β1等多种相关纤维化指标表达,并通过影响组织内磷酸酶和张力蛋白同源物-磷脂酰肌醇3激酶/蛋白激酶B、丝裂原活化蛋白激酶/细胞外调节蛋白激酶、腺苷A2A受体-核因子E2相关因子2/血红素加氧酶1、Wnt/β-catenin等信号通路以实现抗纤维化效果[6,11-12,22-27]。
另外,部分临床研究结果显示,在常规康复疗法的基础上接受体外冲击波疗法的膝关节挛缩患者,其膝关节的主动屈曲角度和Lysholm膝关节功能评分较治疗前或其他未接受体外冲击波疗法的患者均有显著改善,且未观察到不良反应[28-29]。
综上,当前研究更多聚焦于体外冲击波干预在关节挛缩动物模型中的抗纤维化效能,对它在肌肉纤维化组织中的作用涉及较少,仅有2项研究结果证实体外冲击波在肌肉组织中同样具有抗纤维化效果,如表2所示。另外,研究表明体外冲击波可通过增加骨骼肌及关节内部小血管数量、抑制组织内部纤维化相关指标表达、调控相应信号通路等方式对肌源性和关节源性挛缩起抑制作用。然而,目前体外冲击波在肌肉骨骼疾病中的抗纤维化研究多基于动物实验,针对体外冲击波在肌肉纤维化组织及人体中的抗纤维化作用及相关分子信号通路等问题,仍需更多研究予以验证和探索。
2.1.3 脏器疾病
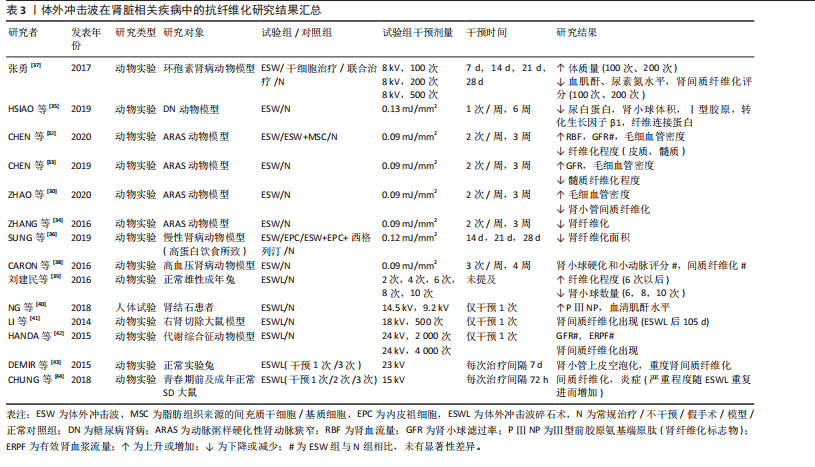
(1)肾脏疾病:动脉粥样硬化性肾动脉狭窄作为一种慢性缺血性肾脏疾病[30],是继发于微血管丧失、纤维化和氧化应激的进行性肾功能恶化,但当前大多数患者在接受肾血管成形术后虽能略微控制肾血管性高血压,但仍不能有效恢复肾功能,这或许与狭窄肾内出现的炎症和纤维化有关[31]。因此,寻找合适且有效治疗肾纤维化的方法对于治疗动脉粥样硬化性肾动脉狭窄至关重要。多项研究结果表明,体外冲击波干预可有效降低动脉粥样硬化性肾动脉狭窄动物模型的肾间质、髓质纤维化程度,促进相应血管内皮生长因子表达,增加毛细血管密度,提高狭窄肾的肾小球滤过率,促进微血管重塑[30,32-34]。
除此之外,相关研究结果显示体外冲击波对糖尿病肾病等多种肾脏疾病有益。糖尿病肾病是一种常见的糖尿病并发症,临床表现主要为肾小球纤维化、Ⅰ型胶原及纤连蛋白生成增加等。HSIAO等[35]运用体外冲击波干预大鼠糖尿病肾病模型,结果显示肾小球纤维化水平虽仍高于正常组,但Ⅰ型胶原、纤连蛋白及转化生长因子β1水平较糖尿病肾病组均已显著降低,肾小球体积减小,蛋白尿问题得到有效改善。炎症反应、纤维化形成等也已被证实是慢性肾病的主要发病机制。SUNG等[36]在由高蛋白饮食所致的慢性肾病动物模型中发现,体外冲击波能有效减少肾脏纤维化面积,同时降低p-Smad3和转化生长因子β的表达,保护慢性肾病大鼠残存的肾脏功能。一项研究结果显示,体外冲击波对环孢素肾病动物模型的干预效果或许与其干预次数的多少密切相关。在接受100次和200次的体外冲击波干预后,大鼠血肌酐、尿素氮及肾间质纤维化评分显著降低,α-平滑肌肌动蛋白、转化生长因子β1等多项纤维化指标表达减少,而500次的体外冲击波干预表现出了相反的效果[37]。CARON等[38]在研究中发现,体外冲击波并不影响高血压肾病动物模型的肾小球硬化、小动脉评分以及
肾间质纤维化程度,这一结果的出现或与造模使用的一氧化氮还原剂有关。
以上相关研究证实了体外冲击波可在一定程度上抑制肾组织纤维化的形成,但体外冲击波碎石术作为泌尿系统结石的一线治疗方法,虽也属体外冲击波疗法,但相较于其他体外冲击波疗法,其干预剂量较大。因此,部分研究者认为体外冲击波碎石术或将促进肾纤维化形成,并在一定程度上影响肾脏的正常功能[39-44]。研究发现,不同强度的体外冲击波碎石术可导致正常动物模型肾脏出现纤维化病理改变,且纤维化严重程度与接受干预的次数密切相关,次数越多,纤维化程度将越严重[39,43-44]。刘建民等[39]证实4次体外冲击波碎石术后,组织内转化生长因子β1及Ⅳ型胶原蛋白的表达会显著增强;6次体外冲击波碎石术后,肾脏内将出现严重且不可逆转的损害。因此,应严格控制同一肾脏体外冲击波碎石术的治疗次数。一项临床研究结果表明所有接受了体外冲击波碎石术患者的肾纤维化标志物(Ⅲ型前胶原氨基端原肽)水平均在治疗1年后达到高峰,2年时逐步恢复至基线水平,且这一变化与体外冲击波碎石术的使用方式无关[40]。
如表3所示,近年体外冲击波在肾脏疾病中的抗纤维化研究结果表明,体外冲击波对动脉粥样硬化性肾动脉狭窄、糖尿病肾病等多种肾病动物模型有益,其主要通过抗纤维化实现恢复肾脏正常功能的目的。然而,高能量的体外冲击波干预(如体外冲击波碎石术)则表现出了相反作用,可促进体内炎症因子及肾纤维化标志物的产生,引发肾损伤,且肾损伤并不会随干预的结束而消失。因此,这一不良反应的出现提示在使用体外冲击波或体外冲击波碎石术的过程中应严格控制干预的剂量和次数,当超出安全范围后,体外冲击波干预将可能促使正常组织出现纤维化病理改变。但由于当前研究者使用的体外冲击波干预方案差异较大,且干预对象多为动物模型,因此仍需更多研究证据对体外冲击波干预的具体量效关系加以验证。
(2)心脏疾病:现有研究显示,由于心肌梗死或其他原因导致心脏内部供血不足时,心肌纤维化形成和梗死心肌内血管减少将引发心脏结构和功能的改变,因此心肌纤维化通常被认为是心力衰竭发生发展的主导因素[1]。现有治疗方法大多仅能改善相应临床症状,而无法避免心肌缺血患者心脏结构的改变[45]。因此,若能及时抑制心肌纤维化产生,预防心室重构,将可极大改善心肌缺血患者的临床预后。如表4所示,心外冲击波通过影响PI3K/Akt、MAPK等信号通路,降低各类心肌梗死动物模型体内α-平滑肌肌动蛋白、Smad3及转化生长因子β等纤维化相关蛋白表达,抑制心肌纤维化形成[45-48]。部分研究者证实体外冲击波干预可上调血管内皮生长因子及其受体的表达,诱导内皮细胞释放含有miR-19a-3p的血管生成外泌体,促进心脏坏死区及边缘区内血管生成,通过RNA/蛋白质复合物的介导,激活Toll样受体3,诱导缺血
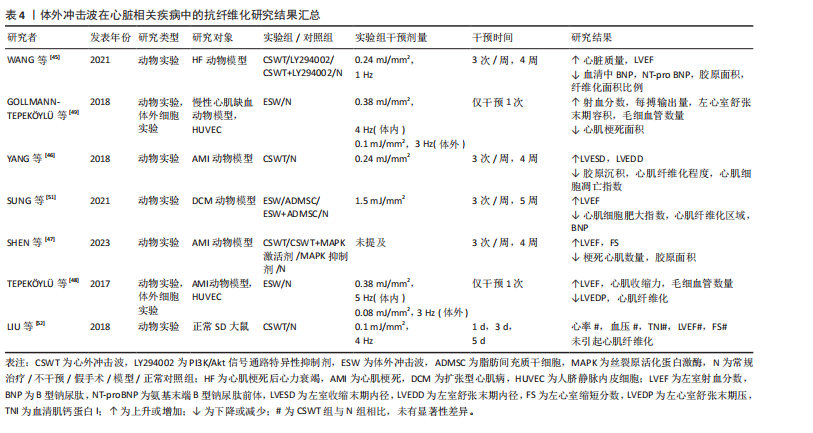
心肌再生,降低心肌纤维化程度,改善左心室功能[48-50]。
除此之外,扩张型心肌病作为一种非缺血相关的心肌疾病,也伴有结构和功能性心肌异常,其心肌组织中同样会出现纤维化这一病理学改变。SUNG等[51]通过一项动物实验发现相较于对照组,仅接受体外冲击波干预的扩张型心肌病大鼠的心肌梗死数量及纤维化面积显著降低,左心室射血分数显著提高,相应心功能得到明显改善。另外,考虑到心外冲击波对组织和细胞具有非选择性,LIU等[52]通过一项动物实验对心外冲击波的安全性进行了评价,研究结果显示心外冲击波组大鼠心肌纤维排列正常,心肌组织内并未出现炎症反应和纤维化改变;同时,根据目前超微结构损伤程度评价体系,心外冲击波组大鼠心肌组织中的微细结构出现了如线粒体肿胀、线粒体嵴结构消失等与对照组相比不存在统计学差异的异常情况,这一改变或将刺激心肌细胞的自我修复,促进心肌内新生血管增加。
综上,基于现有临床前研究证据,心外冲击波在心力衰竭、慢性心肌缺血、心肌梗死和扩张型心肌病动物模型中展现出了显著的抗心肌纤维化形成能力。心外冲击波可经多种途径抑制纤维化相关信号分子表达,促进新血管生成及心肌再生,从而延缓或者直接避免心室病理性重构,改善心肌功能。此外,已有动物实验研究结果证实了心外冲击波的安全性,经心外冲击波干预的正常SD大鼠心肌组织内并未出现病理性损伤,这将为日后临床治疗相应疾病提供新的可能性。
(3)肝硬化:肝纤维化的产生在肝硬化疾病的发生、发展过程中扮演着重要角色,但目前针对肝硬化的治疗手段仅可改善肝脏功能,并不能逆转肝组织纤维化改变。UJIIE等[53]尝试使用体外冲击波干预由四氯化碳诱导形成的药物性大鼠肝硬化模型,研究结果显示体外冲击波可通过抑制转化生长因子β1表达和胶原蛋白的形成,显著降低肝硬化大鼠的肝纤维化面积,同时增强血管内皮生长因子B等血管生成因子表达,促进门静脉周围血管生成,有效改善肝脏功能。
(4)急性呼吸窘迫综合征:长期、持续的肺损伤可能发展为急性呼吸窘迫综合征,患者将出现呼吸窘迫、顽固性低氧血症和呼吸衰竭等严重临床症状,并可在体内观察到肺组织的纤维化改变。一项动物实验研究结果表明,体外冲击波干预可有效改善急性呼吸窘迫综合征大鼠肺实质拥挤评分,提高肺囊泡数量,并通过减少Smad3、转化生长因子β等相关纤维化指标表达而有效降低肺组织纤维化程度及胶原沉积面积,改善肺功能[54]。
(5)子宫内粘连:损伤、感染等因素可导致子宫内膜丧失自我修复和血管生成能力,促使纤维结缔组织取代正常子宫内膜,最终形成子宫壁间粘连。CHENG等[55]发现体外冲击波可有效改善子宫内粘连动物模型的子宫形态,提升子宫内膜腺体数量,并通过下调转化生长因子β、纤维连接蛋白等纤维化标志物的mRNA表达水平降低子宫内膜中胶原纤维和纤维化瘢痕的形成程度,由此证明体外冲击波能在预防和治疗阶段为抗子宫内膜纤维化提供帮助。
(6)前列腺疾病:前列腺组织的纤维化改变可能引发尿急、尿频、排尿困难、性功能障碍等一系列临床症状,严重者将为患者带来巨大的生理和心理压力,增加焦虑、抑郁的风险。目前,不少研究认为体外冲击波的抗纤维化作用或将为前列腺相关疾病的临床治疗提供新思路。SONG等[56]证实体外冲击波干预可通过影响沉默调节蛋白1、核转录因子κB等细胞因子表达,改善慢性非细菌性前列腺炎大鼠的尿路功能障碍,显著降低前列腺的纤维化面积和炎症浸润程度。另外,一项运用体外冲击波疗法治疗药物难治性良性前列腺增生患者的研究结果显示,经8周体外冲击波疗法治疗后,29例患者的国际前列腺症状评分、最大尿流量、残余尿量在治疗和随访期间均得到了显著改善,且无严重不良反应发生[57]。
(7)膀胱疾病:膀胱间质纤维化的形成将在一定程度上引起膀胱功能障碍,临床上通常表现为尿失禁、压力性排尿及尿路感染等症状,严重者将影响患者的生活质量及社会活动。近年相关研究结果表明,对于由放疗及脂多糖诱导的慢性膀胱炎动物模型,体外冲击波干预可显著降低膀胱炎大鼠的膀胱纤维化、胶原沉积面积以及纤维化、炎症指标表达,改善膀胱结构和功能[58-59]。CHEN等[60]运用体外冲击波干预氯胺酮诱导的膀胱功能障碍大鼠,发现体外冲击波具有同样作用,且体外冲击波的能流密度越高,相关指标变化越明显。LIN等[61]证实0.12 mJ/mm2的体外冲击波可使膀胱过度活动大鼠膀胱内的纤维化标志物含量显著降低,0.25 mJ/mm2的体外冲击波可改善膀胱过度活动患者的相应临床症状,显著降低膀胱过度活动症状评分,提高患者生活质量。另外,KAWASE等[62]发现体外冲击波对由脊髓损伤导致的膀胱及尿道功能障碍有益,可有效降低脊髓损伤大鼠尿道纤维化面积,提高损伤大鼠排尿效率,但对降低膀胱纤维化程度无益,这或许与体外冲击波干预的有效范围有关。
体外冲击波在部分脏器疾病中的抗纤维化研究结果见表5。多项动物实验结果表明,对于肝硬化、急性呼吸窘迫综合征、膀胱功能障碍等多种脏器纤维化疾病,体外冲击波可通过调控转化生长因子β/Smads通路中的关键节点、改善组织炎症反应等方式降低肝脏、肺及膀胱等多种组织器官的纤维化程度,促进组织正常结构恢复,进而显著改善相应器官的功能障碍。与此同时,少量人体试验结果已证实体外冲击波疗法在临床抗纤维化治疗中的有效性和安全性。但当前针对体外冲击波干预各类脏器纤维化疾病的基础研究与临床试验数量较少,缺乏对其在不同脏器纤维化病理进程中的分子机制及疗效研究,因此未来还需更多学者对该领域进行研究证实。
2.1.4 其他疾病 除上述疾病外,体外冲击波干预对椎板切除术后的硬膜外纤维化、硅胶器械植入后的包膜纤维化及由放射治疗引起的皮肤纤维化同样有效,见表6。外科术后相关手术区域内出现的组织纤维化病理改变将可能对手术结果产生一定影响,HABERAL等[63]发现体外冲击波在降低L3-L4全椎板切除术后大鼠硬膜外纤维化程度的同时,并未对相关组织内急慢性炎症反应和血管增生产生影响。一项运用体外冲击波干预植入硅胶动物模型的实验结果显示,植入术后立即接受单次体外冲击波干预可减缓大鼠后续包膜纤维化的形成速度,同时术后14 d内接受多次体外冲击波干预可促进包膜纤维化组织的主动降解,显著降低术后100 d的包膜厚度[64]。同样,放射治疗易导致治疗区域皮肤出现纤维化改变,且这一改
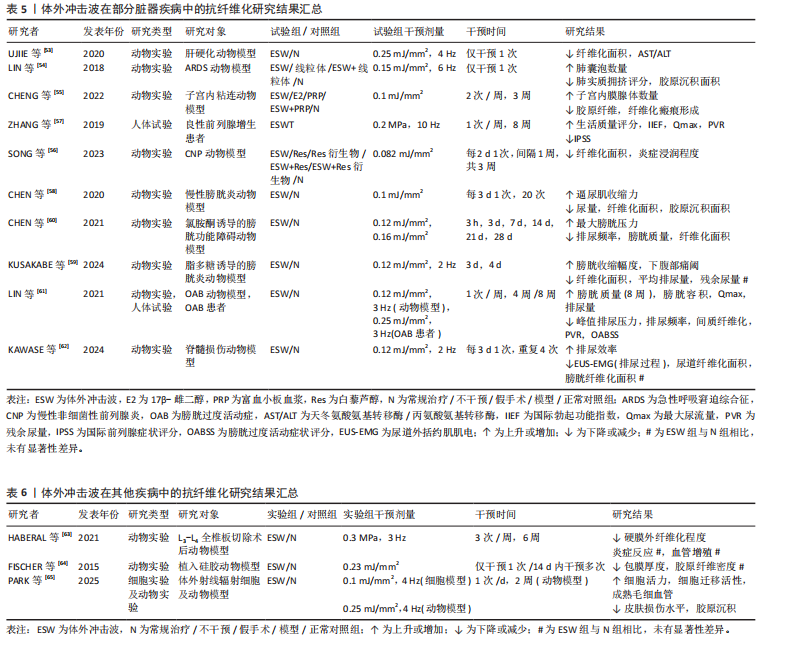
变难以控制和自发消退。PARK等[65]证实体外冲击波干预可提高经射线辐射的人真皮成纤维细胞活力及细胞迁移活性,并通过抑制组织内转化生长因子β1、Smad蛋白表达降低动物模型皮肤损伤程度及胶原沉积面积。上述动物实验研究结果表明,除各类常见的纤维化疾病外,在针对部分由临床治疗引发的组织纤维化改变时,体外冲击波亦可作为一项有效、安全且易于应用的治疗手段以预防和减轻组织纤维化程度,降低相关并发症的发生率。
综上,大量的临床前研究表明,体外冲击波作为一种临床常用的物理治疗方法,可通过力-化学转导机制调控组织内多条与纤维化形成密切相关的分子信号通路,在皮肤病理性瘢痕增生、肌肉骨骼损伤及脏器纤维化病变(包括心肌纤维化、肝纤维化等)等疾病的治疗中表现出显著的抗纤维化作用。另外,当前大多数研究结果均已证实在一定的能流密度范围和干预次数内,体外冲击波干预具有较高的安全性,这为未来开发临床抗纤维化治疗的新方法奠定了研究基础。
2.2 体外冲击波抗纤维化的分子机制 体外冲击波的抗纤维化作用涉及多方面的信号转导通路,且这些通路之间存在一定的相互作用。但截至目前,有关体外冲击波抗纤维化作用的分子机制尚未完全探究清楚,更多发现的是体外冲击波对部分关键因子的调节作用,因此文章将对纳入研究中所提及的信号通路进行初步总结归纳,见图3。
2.2.1 新生血管形成改善组织缺血、缺氧状态 组织缺血可引起内皮细胞功能障碍和周围毛细血管、微血管阻塞,导致缺氧损伤,进而刺激组织纤维化产生[30]。因此,增加纤维组织中的血管密度、促进血管生成是减轻纤维化程度的重要方法。部分研究认为,心外冲击波产生的机械应力可直接作用于缺血心肌,对血管内皮细胞和平滑肌细胞产生生物学效应,进而有效促进组织内新生血管形成[46]。除此之外,大量研究结果显示体外冲击波可上调纤维组织中血管内皮生长因子、成纤维细胞生长因子和内皮一氧化氮合酶等血管生成因子的蛋白表达[34,49,53,55,61],同时其产生的机械刺激还可通过提升典型机械转换器(如局部黏着斑激酶、肝素硫酸酯蛋
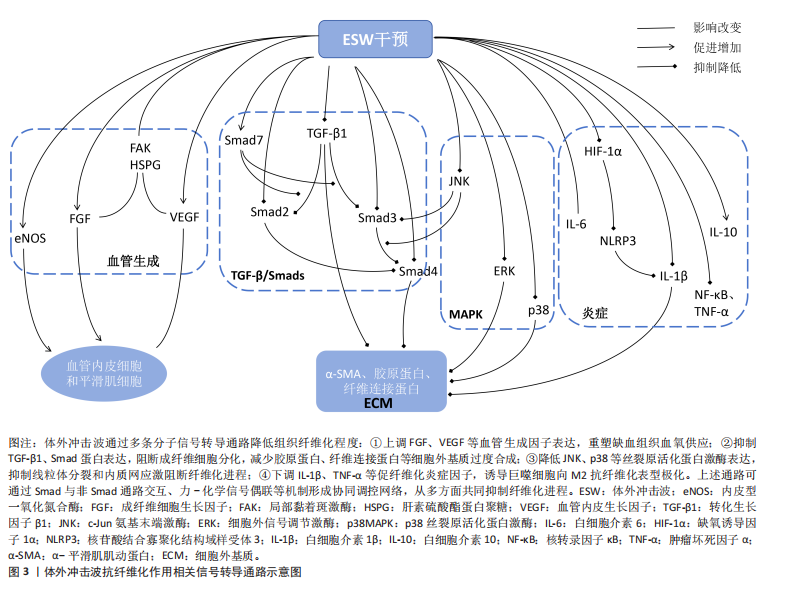
白聚糖)的细胞信号传导功能,促进血管内皮生长因子、成纤维细胞生长因子的产生[33-34,49]。但在慢性环孢素肾病动物模型中,血管内皮生长因子高表达属致病因子,是环孢素诱导肾间质纤维化的重要细胞因子之一,研究结果显示超过一定范围以后,体外冲击波的作用次数越多,动物模型体内血管内皮生长因子表达水平越高,肾间质纤维化程度越严重,由此证明体外冲击波对血管内皮生长因子的影响具有一定的器官差异性[37]。综上,在严格把控体外冲击波的适应证、作用剂量与作用次数的前提下,体外冲击波可通过多种途径共同促进血管生成,改善组织缺血、缺氧状态,以最终实现抗纤维化作用。
2.2.2 转化生长因子β/Smads通路限制细胞外基质异常沉积 相关研究认为,转化生长因子β作为一种影响组织纤维化形成的关键细胞因子,不仅可促使成纤维细胞向具有过度胶原合成能力的肌成纤维细胞分化,还对细胞外基质蛋白合成具有强刺激作用[1,3]。因此,转化生长因子β过表达将导致细胞外基质异常沉积,进而增强组织纤维化程度。而多项研究结果显示,体外冲击波可通过降低组织内转化生长因子β1表达,影响成纤维细胞的分化和肌成纤维细胞的胶原合成,抑制细胞外基质异常沉积[53,58,64,66],或通过直接抑制细胞外基质中α-平滑肌肌动蛋白表达,降低组织纤维化面积[14,67]。
另外,在肌成纤维细胞中,转化生长因子β诱导的细胞外基质表达还受多种Smad蛋白水平的调控。多项研究结果表明,体外冲击波干预可通过抑制多种动物模型体内转化生长因子β、Smad2、Smad3和Smad4蛋白表达,提升组织内Smad7蛋白表达而降低相关组织纤维化程度[20,22,36,58];同时,PARK等[65]发现不同能流密度的体外冲击波可对不同模型的Smad蛋白表达产生不同影响。综上,体外冲击波可通过影响转化生长因子β/Smads通路中多种细胞因子的表达水平而限制组织内细胞外基质的异常沉积,降低组织纤维化程度。
2.2.3 MAPK通路经多种子通路抑制纤维化形成 MAPK通路在组织纤维化、炎症反应、细胞分化,增殖和凋亡中均具有关键调节作用[47]。有研究结果表明,c-Jun氨基末端激酶(c-Jun N-terminal kinase,JNK)过度激活可影响转化生长因子β1的生物学活性,进而通过刺激转化生长因子β/Smad等信号通路,加速线粒体分裂和内质网应激促进纤维化进程[12]。但多项研究结果显示经体外冲击波干预后,各动物模型体内p-JNK/JNK蛋白的表达水平将显著降低[12,58,61],Smad3蛋白磷酸化过程及Smad3与Smad4之间的相互作用也将受到影响[12],这些变化可在一定程度上抑制组织纤维化的发展;其次,部分研究者发现体外冲击波可通过抑制细胞外信号调节激酶与p38蛋白表达[25,47,58],控制成纤维细胞增殖、胶原蛋白生成和细胞外基质重塑。综上,经体外冲击波干预后,MAPK通路可经多种途径影响转化生长因子β/Smad信号通路表达和细胞外基质重塑,进而抑制组织纤维化形成。但经体外冲击波干预后各通路的具体上下游蛋白、子通路间是否存在相互作用等问题仍需未来进一步探究。
2.2.4 炎症因子改变降低组织纤维化程度 在纤维化形成过程中,持续的炎症反应将触发巨噬细胞迁移和成纤维细胞的增殖分化,导致细胞外基质过度形成及组织内胶原蛋白的过量沉积[23,26,58,64]。多项研究结果显示,体外冲击波干预可有效减少巨噬细胞浸润[11,64],并促使巨噬细胞表型由M1向M2转化[33]。其中,M1型巨噬细胞具有促炎作用,与组织中白细胞介素6、白细胞介素1β、肿瘤坏死因子α及核转录因子κB等相关促炎因子水平密切相关[25,35]。研究结果证实,体外冲击波干预可显著降低各动物模型体内促炎因子的蛋白表达水平,抑制组织炎症反应[35,55-56,60]。
但IWATSU等[11]发现体外冲击波干预可通过诱导机械转导途径,上调膝关节屈曲挛缩动物模型体内白细胞介素6水平而抑制炎症反应。因此,未来对于白细胞介素6在纤维化形成中的角色仍需继续探讨。
另一方面,具有抗炎特性的M2型巨噬细胞主要与组织中白细胞介素10等抗炎因子表达有关。HSIAO等[35]的研究结果显示,经体外冲击波干预后糖尿病肾病动物模型体内的白细胞介素10含量显著升高。另外,有研究认为NLRP3炎症小体是驱动大多数纤维化疾病的病理学核心,经缺氧诱导因子1α激活后可促进白细胞介素1β释放,进而将器官中的内在细胞转化为成纤维细胞并产生大量胶原蛋白,最终介导组织纤维化形成。而体外冲击波干预可有效抑制该通路,显著降低缺氧诱导因子1α、NLRP3及白细胞介素1β的蛋白表达,减轻膝关节伸直挛缩动物模型的骨骼肌纤维化程度[23]。综上,体外冲击波可通过减少巨噬细胞浸润、转变巨噬细胞表型和直接改变组织中多种炎症因子表达水平而降低组织纤维化程度。但目前对于各炎症因子在纤维化形成过程中的相互作用尚未明确,还需未来的进一步研究。
除此之外,体外冲击波还可通过其他细胞因子或信号通路抑制组织纤维化形成,例如基质金属蛋白酶[16,46,64]、ID蛋白[66]、PI3K/Akt信号通路[24,45]、A2AR-Nrf2/HO-1信号通路及黏着斑信号通路等[26,46]。但目前在这些方面的研究数量较少,具体作用仍不明确,还需未来更多研究加以证实。
综上所述,血管生成因子、转化生长因子β/Smads通路、MAPK通路和炎症因子可能是目前体外冲击波降低组织纤维化程度的统一分子机制,这些信号通路经体外冲击波干预后可在恢复组织缺血、缺氧状态的同时,抑制成纤维细胞分化、胶原蛋白产生及细胞外基质过度沉积,实现抗纤维化,这将在一定程度上为未来临床体外冲击波抗纤维化治疗提供理论基础,但目前仅较为明确体外冲击波对部分关键因子(如转化生长因子β1)的上下调作用,在对于体外冲击波抗纤维化的整体分子机制研究方面仍存在较大空白。因此,未来探究体外冲击波抗纤维化的分子机制仍需向特殊化、整体化的方向发展。

| [1] LURJE I, GAISA NT, WEISKIRCHEN R, et al. Mechanisms of organ fibrosis: Emerging concepts and implications for novel treatment strategies. Mol Aspects Med. 2023;92:101191. [2] KU JC, RAITEN J, LI Y. Understanding fibrosis: Mechanisms, clinical implications, current therapies, and prospects for future interventions. Biomed Eng Adv. 2024;7:100118. [3] YOUNESI FS, MILLER AE, BARKER TH, et al. Fibroblast and myofibroblast activation in normal tissue repair and fibrosis. Nat Rev Mol Cell Biol. 2024;25(8):617-638. [4] DEMUYNCK L, MOONEN S, THIESSEN F, et al. Systematic Review on Working Mechanisms of Signaling Pathways in Fibrosis During Shockwave Therapy. Int J Mol Sci. 2024;25(21):11729. [5] DARBY IA, LAVERDET B, BONTÉ F, et al. Fibroblasts and myofibroblasts in wound healing. Clin Cosmet Investig Dermatol. 2014;7:301-311. [6] ZHOU Y, YANG K. Prevention of arthrofibrosis during knee repair by extracorporeal shock wave therapy: Preliminary study in rabbits. Injury. 2019;50(3):633-638. [7] JESCHKE MG, WOOD FM, MIDDELKOOP E, et al. Scars. Nat Rev Dis Primers. 2023;9(1):64. [8] D’AGOSTINO MC, CRAIG K, TIBALT E, et al. Shock wave as biological therapeutic tool: From mechanical stimulation to recovery and healing, through mechanotransduction. Int J Surg. 2015;24(Pt B):147-153. [9] 梁豪君,贾海光,朱俊宇,等.中国骨肌疾病体外冲击波疗法指南(2023年版)[J].中国医学前沿杂志(电子版),2023,15(9):1-20. [10] 程志祥,樊肖冲,冯智英,等.体外冲击波疗法临床应用中国疼痛学专家共识(2023版)[J].中华疼痛学杂志,2023, 19(2):220-235. [11] IWATSU J, YABE Y, KANAZAWA K, et al. Extracorporeal shockwave therapy in an immobilized knee model in rats prevents progression of joint contracture. J Orthop Res. 2023;41(5):951-961. [12] HU C, ZHANG QB, WANG F, et al. The effect of extracorporeal shock wave on joint capsule fibrosis in rats with knee extension contracture: a preliminary study. Connect Tissue Res. 2023;64(5):469-478. [13] ROQUES C. Scars, physiology, classification and assessment. Soins. 2013;(772):30-33. [14] ZHAO JC, ZHANG BR, HONG L, et al. Extracorporeal shock wave therapy with low-energy flux density inhibits hypertrophic scar formation in an animal model. Int J Mol Med. 2018;41(4):1931-1938. [15] LEE SY, JOO SY, CHO YS, et al. Effect of extracorporeal shock wave therapy for burn scar regeneration: A prospective, randomized, double-blinded study. Burns. 2021;47(4):821-827. [16] WANG CJ, KO JY, CHOU WY, et al. Extracorporeal shockwave therapy for treatment of keloid scars. Wound Repair Regen. 2018;26(1):69-76. [17] KIM DH, HAN SH, SUH HS, et al. Benefits of extracorporeal shock waves for keloid treatment: A pilot study. Dermatol Ther. 2020;33(4):e13653. [18] SAGGINI R, SAGGINI A, SPAGNOLI AM, et al. Extracorporeal Shock Wave Therapy: An Emerging Treatment Modality for Retracting Scars of the Hands. Ultrasound Med Biol. 2016;42(1):185-195. [19] HUANG PP, ZHANG QB, ZHOU Y, et al. Effect of Radial Extracorporeal Shock Wave Combined With Ultrashort Wave Diathermy on Fibrosis and Contracture of Muscle. Am J Phys Med Rehabil. 2021; 100(7):643-650. [20] YIN TC, WU RW, SHEU JJ, et al. Combined Therapy with Extracorporeal Shock Wave and Adipose-Derived Mesenchymal Stem Cells Remarkably Improved Acute Ischemia-Reperfusion Injury of Quadriceps Muscle. Oxid Med Cell Longev. 2018;2018:6012636. [21] MARINELLI L, MORI L, SOLARO C, et al. Effect of radial shock wave therapy on pain and muscle hypertonia: a double-blind study in patients with multiple sclerosis. Mult Scler. 2015;21(5):622-629. [22] LI Y, LIAO Q, ZENG J, et al. Extracorporeal Shock Wave Therapy Improves Nontraumatic Knee Contracture in a Rat Model. Clin Orthop Relat Res. 2023;481(4): 822-834. [23] 肖亚茹.体外冲击波治疗通过对HIF-1α、NLRP3表达的调控减轻大鼠膝关节伸直固定诱导的肌源性挛缩[D].合肥:安徽医科大学,2023. [24] ZHANG R, ZHANG R, ZHOU T, et al. Preliminary investigation on the effect of extracorporeal shock wave combined with traction on joint contracture based on PTEN-PI3K/AKT pathway. J Orthop Res. 2024;42(2):339-348. [25] ZHANG R, ZHANG QB, ZHOU Y, et al. Possible mechanism of static progressive stretching combined with extracorporeal shock wave therapy in reducing knee joint contracture in rats based on MAPK/ERK pathway. Biomol Biomed. 2023;23(2):277-286. [26] YUAN H, WANG K, ZHANG QB, et al. The effect of extracorporeal shock wave on joint capsule fibrosis based on A2AR-Nrf2/HO-1 pathway in a rat extending knee immobilization model. J Orthop Surg Res. 2023;18(1):930. [27] HUO L, ZHANG QB, ZHU DT, et al. Preliminary study of extracorporeal shock wave alleviating joint capsule fibrosis caused by internal bleeding of knee joint in rats. Connect Tissue Res. 2024;65(5):397-406. [28] 张润,张全兵,周云,等.不同强度体外冲击波治疗创伤性伸直型膝关节挛缩的疗效分析[J].中国康复医学杂志,2024, 39(11):1625-1631. [29] 王锋,张全兵,周云,等.发散式冲击波联合常规康复治疗创伤后膝关节伸直挛缩的疗效观察[J].中国骨与关节损伤杂志,2020,35(2):187-189. [30] ZHAO Y, SANTELLI A, ZHU XY, et al. Low-Energy Shockwave Treatment Promotes Endothelial Progenitor Cell Homing to the Stenotic Pig Kidney. Cell Transplant. 2020;29:963689720917342. [31] KWON SH, LERMAN LO. Atherosclerotic renal artery stenosis: current status. Adv Chronic Kidney Dis. 2015;22(3):224-231. [32] CHEN XJ, ZHANG X, JIANG K, et al. Adjunctive mesenchymal stem/stromal cells augment microvascular function in poststenotic kidneys treated with low-energy shockwave therapy. J Cell Physiol. 2020;235(12):9806-9818. [33] CHEN XJ, ZHANG X, JIANG K, et al. Improved renal outcomes after revascularization of the stenotic renal artery in pigs by prior treatment with low-energy extracorporeal shockwave therapy. J Hypertens. 2019;37(10): 2074-2082. [34] ZHANG X, KRIER JD, AMADOR CARRASCAL C, et al. Low-Energy Shockwave Therapy Improves Ischemic Kidney Microcirculation. J Am Soc Nephrol. 2016; 27(12):3715-3724. [35] HSIAO CC, HUANG WH, CHENG KH, et al. Low-Energy Extracorporeal Shock Wave Therapy Ameliorates Kidney Function in Diabetic Nephropathy. Oxid Med Cell Longev. 2019;2019:8259645. [36] SUNG PH, CHEN KH, LI YC, et al. Sitagliptin and shock wave-supported peripheral blood derived endothelial progenitor cell therapy effectively preserves residual renal function in chronic kidney disease in rat-role of dipeptidyl peptidase 4 inhibition. Biomed Pharmacother. 2019;111:1088-1102. [37] 张勇.低能量冲击波和骨髓间充质干细胞对慢性环孢素肾病大鼠的治疗研究[D].上海:中国人民解放军海军军医大学, 2017. [38] CARON J, MICHEL PA, DUSSAULE JC, et al. Extracorporeal shock wave therapy does not improve hypertensive nephropathy. Physiol Rep. 2016;4(11):e12699. [39] 刘建民,李振,汪盛,等.重复体外冲击波碎石引起兔肾组织结构的变化研究[J].中华全科医学,2016,14(12):2027-2030. [40] NG CF, LUKE S, YEE CH, et al. Extracorporeal Shockwave Lithotripsy Could Lead to a Prolonged Increase in the Renal Fibrotic Process of Up to 2 Years. J Endourol. 2018; 32(3):223-229. [41] LI X, LONG Q, CHENG X, et al. Shock wave induces biological renal damage by activating excessive inflammatory responses in rat model. Inflammation. 2014;37(4):1317-1325. [42] HANDA RK, JOHNSON CD, CONNORS BA, et al. Shock wave lithotripsy does not impair renal function in a Swine model of metabolic syndrome. J Endourol. 2015; 29(4):468-473. [43] DEMIR A, TÜRKER P, BOZKURT SU, et al. The histomorphological findings of kidneys after application of high dose and high-energy shock wave lithotripsy. Cent European J Urol. 2015;68(1):72-78. [44] CHUNG JM, PARK BK, KIM JH, et al. Impact of repeated extracorporeal shock wave lithotripsy on prepubertal rat kidney. Urolithiasis. 2018;46(6):549-558. [45] WANG L, TIAN X, CAO Y, et al. Cardiac Shock Wave Therapy Improves Ventricular Function by Relieving Fibrosis Through PI3K/Akt Signaling Pathway: Evidence From a Rat Model of Post-infarction Heart Failure. Front Cardiovasc Med. 2021;8:693875. [46] YANG W, HE Y, GAN L, et al. Cardiac shock wave therapy promotes arteriogenesis of coronary micrangium, and ILK is involved in the biomechanical effects by proteomic analysis. Sci Rep. 2018;8(1):1814. [47] SHEN Y, LUO Z, ZHONG D, et al. Cardiac Shock Wave Treatment Enhances Myocardial Function in Rat Model of Myocardial Infarction by Regulating MAPK Signaling Pathway. J Biol Regul Homeost Agents. 2023;37(7):3751-3760. [48] TEPEKÖYLÜ C, PRIMESSNIG U, PÖLZL L, et al. Shockwaves prevent from heart failure after acute myocardial ischaemia via RNA/protein complexes. J Cell Mol Med. 2017;21(4): 791-801. [49] GOLLMANN-TEPEKÖYLÜ C, LOBENWEIN D, THEURL M, et al. Shock Wave Therapy Improves Cardiac Function in a Model of Chronic Ischemic Heart Failure: Evidence for a Mechanism Involving VEGF Signaling and the Extracellular Matrix. J Am Heart Assoc. 2018;7(20):e010025. [50] GOLLMANN-TEPEKÖYLÜ C, PÖLZL L, GRABER M, et al. miR-19a-3p containing exosomes improve function of ischaemic myocardium upon shock wave therapy. Cardiovasc Res. 2020;116(6):1226-1236. [51] SUNG PH, LEE MS, CHAI HT, et al. Extracorporeal Shock Wave Enhanced Exogenous Mitochondria into Adipose-Derived Mesenchymal Stem Cells and Further Preserved Heart Function in Rat Dilated Cardiomyopathy. Biomedicines. 2021;9(10):1362. [52] LIU B, ZHANG Y, JIA N, et al. Study of the Safety of Extracorporeal Cardiac Shock Wave Therapy: Observation of the Ultrastructures in Myocardial Cells by Transmission Electron Microscopy. J Cardiovasc Pharmacol Ther. 2018;23(1):79-88. [53] UJIIE N, NAKANO T, YAMADA M, et al. Low-energy extracorporeal shock wave therapy for a model of liver cirrhosis ameliorates liver fibrosis and liver function. Sci Rep. 2020;10(1):2405. [54] LIN KC, WALLACE CG, YIN TC, et al. Shock Wave Therapy Enhances Mitochondrial Delivery into Target Cells and Protects against Acute Respiratory Distress Syndrome. Mediators Inflamm. 2018;2018:5425346. [55] CHENG YH, TSAI NC, CHEN YJ, et al. Extracorporeal Shock Wave Therapy Combined with Platelet-Rich Plasma during Preventive and Therapeutic Stages of Intrauterine Adhesion in a Rat Model. Biomedicines. 2022;10(2):476. [56] SONG Z, JIN C, BIAN Z, et al. Radial Extracorporeal Shock Wave Therapy Combined with Resveratrol Derivative Alleviates Chronic Nonbacterial Prostatitis in Rats. Inflammation. 2023; 46(2):584-597. [57] ZHANG D, WANG YL, GONG DX, et al. Radial Extracorporeal Shock Wave Therapy as a Novel Agent for Benign Prostatic Hyperplasia Refractory to Current Medical Therapy. Am J Mens Health. 2019;13(1): 1557988319831899. [58] CHEN YT, YANG CC, SUNG PH, et al. Long-term effect of extracorporeal shock wave therapy on attenuating radiation-induced chronic cystitis in rat. Am J Transl Res. 2020; 12(3):999-1015. [59] KUSAKABE N, KAMIJO TC, WADA N, et al. Effects of low-intensity extracorporeal shock wave therapy on lipopolysaccharide cystitis in a rat model of interstitial cystitis/bladder pain syndrome. Int Urol Nephrol. 2024;56(1):77-86. [60] CHEN YT, HUANG KH, CHIANG JY, et al. Extracorporeal Shock Wave Therapy Protected the Functional and Architectural Integrity of Rodent Urinary Bladder against Ketamine-Induced Damage. Biomedicines. 2021;9(10):1391. [61] LIN KL, LU JH, CHUEH KS, et al. Low-Intensity Extracorporeal Shock Wave Therapy Promotes Bladder Regeneration and Improves Overactive Bladder Induced by Ovarian Hormone Deficiency from Rat Animal Model to Human Clinical Trial. Int J Mol Sci. 2021;22(17): 9296. [62] KAWASE K, KAMIJO TC, KUSAKABE N, et al. Effects of low-intensity extracorporeal shock wave on bladder and urethral dysfunction in spinal cord injured rats. Int Urol Nephrol. 2024;56(12):3773-3781. [63] HABERAL B, ŞIMŞEK EK, AKPINAR K,et al. Impact of radial extracorporeal shock wave therapy in post-laminectomy epidural fibrosis in a rat model. Jt Dis Relat Surg. 2021;32(1):162-169. [64] FISCHER S, MUELLER W, SCHULTE M, et al. Multiple extracorporeal shock wave therapy degrades capsular fibrosis after insertion of silicone implants. Ultrasound Med Biol. 2015;41(3):781-789. [65] PARK SW, SHIN J, JEONG BK, et al. The Effects of Extracorporeal Shock Wave Therapy on Cutaneous Radiation Injury in a Mouse Model. Plast Reconstr Surg. 2025;155(5):813-825. [66] CUI HS, HONG AR, KIM JB, et al. Extracorporeal Shock Wave Therapy Alters the Expression of Fibrosis-Related Molecules in Fibroblast Derived from Human Hypertrophic Scar. Int J Mol Sci. 2018;19(1):124. [67] RINELLA L, MARANO F, BERTA L, et al. Extracorporeal shock waves modulate myofibroblast differentiation of adipose-derived stem cells. Wound Repair Regen. 2016;24(2):275-286. |
| [1] | 侯超文, 李兆进, 孔健达, 张树立. 骨骼肌衰老主要生理变化及运动的多机制调控作用[J]. 中国组织工程研究, 2026, 30(6): 1464-1475. |
| [2] | 刘可新, 郝凯敏, 庄文越, 李正祎. 自噬相关基因在肺纤维化模型中的表达:生物信息学分析及实验验证[J]. 中国组织工程研究, 2026, 30(5): 1129-1138. |
| [3] | 李郝静, 王 新, 宋成林, 张胜男, 陈云昕. 上斜方肌处体外冲击波与运动控制训练治疗慢性非特异性颈痛[J]. 中国组织工程研究, 2026, 30(5): 1162-1170. |
| [4] | 余慧芬, 莫李存, 程乐平. 5 -羟色胺在组织损伤修复中的地位与角色[J]. 中国组织工程研究, 2026, 30(5): 1196-1206. |
| [5] | 余诗宇, 俞苏桐, 徐 杨, 镇祥燕, 韩凤选. 组织工程治疗策略在口腔黏膜下纤维化中的研究与应用进展[J]. 中国组织工程研究, 2026, 30(4): 936-948. |
| [6] | 暨凯忠, 孔一豪, 支忆清, 金莹莹, 陈建权. 锌指DHHC型棕榈酰转移酶5在组织稳态和疾病中的作用及机制[J]. 中国组织工程研究, 2026, 30(17): 4430-4445. |
| [7] | 彭 皓, 蒋 阳, 宋艳萍, 吴 泉, 姚 娜, 陈奇刚, 申 震. 不同骨骼疾病动物模型中H型血管的生成及作用[J]. 中国组织工程研究, 2026, 30(16): 4154-4165. |
| [8] | 付 晓, 李纪高, 闫小楠, 宋 哲, 郭岳峻, 李韩冰, 周 全. 中药有效成分治疗类风湿关节炎:基于核因子κB信号通路的机制[J]. 中国组织工程研究, 2026, 30(16): 4180-4192. |
| [9] | 封 涛, 殷照阳. 股骨转子间骨折外侧壁完整性的生物力学功能及临床意义[J]. 中国组织工程研究, 2026, 30(15): 3960-3970. |
| [10] | 黄 磊, 王向红, 张先绪, 李世成, 罗志强. 核因子E2相关因子2调控非感染性脊柱疾病的机制与治疗潜力[J]. 中国组织工程研究, 2026, 30(15): 3971-3982. |
| [11] | 韩亚澎, 高 俊, 钮云伟, 邓恩甲. 骨碎补总黄酮介导骨相关细胞程序性死亡的机制[J]. 中国组织工程研究, 2026, 30(12): 3091-3099. |
| [12] | 洪润洋, 周琦玥, 范臻成, 时语婕, 陈 昊, 潘 春. 孕期低剂量六氟环氧丙烷二聚酸暴露对子代小鼠肾脏毒性的影响机制[J]. 中国组织工程研究, 2026, 30(11): 2752-2763. |
| [13] | 姚顺华, 黄彩丁, 张梦玉, 张可馨, 尹长江, 杨坤宝. 灰兜巴抑制高糖培养HK-2细胞的铁死亡减轻细胞纤维化[J]. 中国组织工程研究, 2026, 30(11): 2774-2783. |
| [14] | 毛苏杰, 高 洁, 潘壮丽. 免疫细胞在训练诱导应激应答中协同调节炎症反应、肌肉再生和代谢稳态[J]. 中国组织工程研究, 2026, 30(10): 2671-2680. |
| [15] | 焦太强, 韩兴稷, 李向阳, 南 一, 袁 玲, 李佳庆, 牛 阳. 麻杏苦甘汤干预油酸诱导大鼠急性肺损伤的作用机制[J]. 中国组织工程研究, 2026, 30(10): 2430-2439. |
体外冲击波疗法(extracorporeal shock wave therapy,ESWT)作为一种非侵入性的物理治疗方法,因无创、无不良反应、可重复性好、患者耐受性和依从性好等优点被广泛运用于不同医学领域,其中包括骨科学、康复医学、皮肤病学、神经病学等[8]。冲击波是一种兼具声学、力学及光学的声波脉冲(机械波),其产生的机械刺激可引起介质的压强、温度、密度等物理性质发生跳跃式改变,对人体组织产生机械效应、空化效应和热效应[9],进而引发组织细胞的各类生物反应,实现治疗目的。近年来,体外冲击波疗法的临床适应证已突破传统肌肉骨骼系统疾病的范畴,除广泛应用于骨不连、肱骨外上髁炎及慢性肌腱病等经典适应证外,还应用于神经病理性疼痛(如带状疱疹后神经痛)、心血管疾病(如缺血性心脏病/心肌纤维化)等领域。值得关注的是,体外冲击波疗法还可与富血小板血浆、脉冲射频治疗等其他治疗手段联合使用,为更多复杂疾病的治疗提供了更优解[10]。除此之外,研究发现体外冲击波所产生的机械刺激可通过多种途径改变组织细胞内多种生物因子表达,抑制纤维化形成,进而改善患者的临床症状[11-12]。
文章通过检索筛选近10年有关体外冲击波抑制纤维化形成的原创性研究,回顾体外冲击波干预对纤维化形成的影响,探究体外冲击波抗纤维化潜在的分子机制。一方面,通过总结多项实验结果,阐述体外冲击波在纤维组织中的应用价值;另一方面,通过归纳总结有关体外冲击波抗纤维化的分子信号转导通路,以期更好地开发体外冲击波在抑制纤维化形成中的作用,为后续临床治疗纤维化疾病提供新的参考。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
1.1 资料来源
1.1.1 检索人及检索时间 第一作者于2024年10月进行检索。
1.1.2 检索文献时限 2014年1月至2024年9月。
1.1.3 检索数据库 PubMed、Web of Science、中国知网、万方及维普数据库。
1.1.4 检索词 以“extracorporeal shockwave therapy,fibrosis”为主题词,以“extracorporeal shockwave therapy,shock wave therapy,shock wave”等,“fibrosis,fibroses”等共10个为自由词检索PubMed、Web of Science数据库;以“冲击波,体外冲击波疗法,纤维化,抗纤维化”为中文检索词检索中国知网、万方及维普数据库。
1.1.5 检索文献类型 研究原著、学位论文、综述及荟萃分析。
1.1.6 检索策略 以PubMed和中国知网为例,具体检索策略见图1。
1.1.7 手工检索情况 无。
1.1.8 检索文献量 PubMed数据库检索得到文献121篇,Web of Science数据库检索得到文献172篇;中国知网检索得到文献27篇,万方数据库检索得到文献31篇,维普数据库检索得到文献12篇。总计363篇文献,英文文献293篇,中文文献70篇。
1.2 入组标准
1.2.1 纳入标准 ①文献研究类型为已发表的、可获取全文的、探究体外冲击波抗纤维化应用及相关分子机制的临床或临床前研究;②研究对象为正常动物模型或人工制造的代谢性、药物性或创伤性的纤维化病理模型,也可为出现纤维化病理改变的患者,不考虑疾病种类、模型诱导严重程度等因素;③研究结果为检测研究对象的临床症状改善或在组织、分子信号转导机制方面发生的纤维化病理改变。
1.2.2 排除标准 ①重复文献;②综述、会议及科普类文章;③与研究主题相关性不高的文献。
1.3 文献质量评估和数据的提取 通过数据库检索共获取文献363篇,去除重复文献122篇,阅读题目、摘要后去除与文章主题无关文献154篇,阅读全文后进一步排除20篇文献,最终纳入文献67篇,具体文献筛选流程见图2。
3.1 既往他人在该领域研究的贡献和存在的问题 当前在体外冲击波抗纤维化的研究中,研究者们已从细胞、动物及临床试验层面证实体外冲击波抑制组织纤维化形成的效果,其中不少临床前研究也已对体外冲击波抗纤维化作用的分子机制进行了初步探索。研究指出,体外冲击波干预可通过影响转化生长因子β/Smads、MAPK及炎症等信号转导通路,有效抑制组织纤维化的过度发展。然而,由于目前研究中普遍存在干预对象、体外冲击波类型、体外冲击波干预方案异质性高的问题,使得不同研究中的组织信号转导出现差异,导致体外冲击波对相关组织产生不同甚至是矛盾的作用。此外,组织纤维化的形成往往是一个复杂的过程,是多种生物反应综合作用的结果。但当前相关研究大多只探讨了体外冲击波对纤维组织中某一分子通路的影响,探索方向较为单一。因此,若要将体外冲击波的抗纤维化作用应用于临床实践中,还需开展更为全面深入的研究。
3.2 作者综述区别于他人他篇的特点 在以往的综述中,研究者们通常以特定的纤维化相关疾病作为研究体外冲击波抗纤维化作用的切入点,通过探讨体外冲击波在疾病中的作用,以证实体外冲击波对组织纤维化过度形成的抑制作用。然而,体外冲击波的应用范围并不仅局限于单一疾病。此外,DEMUYNCK等[4]更多聚焦于体外冲击波疗法在纤维化组织中的机械转导机制,总结探讨了不同解剖来源纤维化组织对体外冲击波疗法的特异性响应,并归纳了不同能量参数的体外冲击波可能对纤维化组织产生的差异性影响,但存在研究范围较为局限、缺乏随机对照试验及动物实验数据支持,这将对系统性探究体外冲击波抗纤维化的动态分子调节机制、明确体外冲击波疗法抗纤维化的安全性造成一定干扰。此综述从多种纤维化疾病的视角出发,纳入体外细胞实验、动物实验及人体试验等多类原创性研究,较为全面地阐述了体外冲击波抗纤维化的研究进展,并归纳总结了与体外冲击波抗纤维化相关的多条分子信号通路,为后续深入探究体外冲击波抗纤维化的整体信号转导网络,以及验证体外冲击波抗纤维化临床应用的可行性奠定了一定的研究基础。
3.3 综述的局限性 首先,尽管此综述首次依据疾病种类对体外冲击波的抗纤维化效果进行了系统性地归纳与总结,但不同疾病所引用的参考文献数量存在较大差异,这可能会对确定体外冲击波抗纤维化的具体适应证造成干扰;其次,目前有关体外冲击波抗纤维化作用的研究大多停留在基础研究阶段,缺乏足够的临床数据支持;另外,尽管目前研究者们已对体外冲击波的定义达成广泛共识,但各项研究在体外冲击波的类型、干预能量强度及干预时间等方面仍存在显著差异,此综述未能明确体外冲击波在抗纤维化治疗中的量效关系及确立标准化的干预方案;最后,鉴于目前大多数实验仅聚焦于体外冲击波在抗纤维化过程中对单一分子通路的调节作用,未涉及与其他分子通路的相互作用,此综述未能全面阐明体外冲击波抗纤维化的整体分子作用体系。
3.4 综述的重要意义 文章通过对近10年有关体外冲击波抗纤维化实验研究的综合分析,首先依据疾病类型对体外冲击波在纤维组织中的作用进行了回顾。研究发现,体外冲击波在病理性瘢痕(n=5)、关节挛缩(n=12)、肌肉纤维化(n=2)及各类内脏纤维化(n=24)等多类疾病中均表现出显著抗纤维化效能,且体外冲击波的抗纤维化能力与其干预强度及次数呈正相关。但值得注意的是,当干预能量超出适当范围时,高强度的体外冲击波干预(如体外冲击波碎石术)可能导致正常组织出现纤维化病理改变;其次,各项机制研究结果表明,体外冲击波通过促进血管生成因子生成、抑制转化生长因子β/Smad通路关键节点、调节MAPK蛋白表达及改善组织炎症环境等方式,多维度协同抑制成纤维细胞活化及胶原蛋白、细胞外基质的过度沉积。因此,文章不仅为未来临床治疗纤维化疾病提供了新参考,即体外冲击波疗法或许可有效减缓纤维化疾病的发展进程,减轻患者临床症状;同时有利于推动后续体外冲击波抗纤维化标准干预方案的制定,为深入探究体外冲击波抗纤维化的整体分子作用体系奠定基础。
3.5 课题组专家对未来的建议 随着对体外冲击波研究的不断深入,其在纤维化相关疾病中的作用已逐渐被揭示,此综述建议未来研究可着重于以下几个方面:首先,应开展更多大规模、多中心的临床随机对照试验,以验证体外冲击波疗法在不同纤维化疾病中的安全性和有效性;其次,应深入探讨体外冲击波抗纤维化的分子机制,特别是其对特定信号通路的调控作用,以便为临床治疗提供更为精准的理论依据;此外,建议制定统一的体外冲击波抗纤维化治疗标准和操作指南,以规范治疗流程,提高治疗效果的一致性;最后,应加强更多的跨学科合作,整合如生物力学、生物信息学等不同专业领域的先进技术,共同推动体外冲击波抗纤维化治疗技术的发展。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
体外冲击波作为一种高能声波脉冲,可通过机械转导机制调控细胞微环境,诱导多重生物学效应,为临床治疗众多疾病提供新方法。近年来多项研究表明,体外冲击波在纤维化相关疾病的治疗中展现出了显著的生物学调控潜力,其通过可控的机械应力刺激,可有效干预与纤维化进程密切相关的分子及细胞网络。文章旨在突破以往仅聚焦于体外冲击波对纤维化疾病的影响或体外冲击波抗纤维化信号转导通路的研究局限,从多种纤维化相关疾病角度,系统综述了体外冲击波作为抗纤维化疗法的最新研究进展及其潜在的分子机制,为体外冲击波抗纤维化临床研究提供参考依据。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||