中国组织工程研究 ›› 2025, Vol. 29 ›› Issue (34): 7393-7404.doi: 10.12307/2025.483
• 生物材料综述 biomaterial review • 上一篇 下一篇
聚乳酸-羟基乙酸共聚物在口腔医学领域的应用
吴子炜,罗诒财,韦银格,廖红兵
- 广西医科大学口腔医学院/附属口腔医院口腔修复科,广西口腔颌面修复与重建研究重点实验室,广西壮族自治区南宁市 530021
-
收稿日期:2024-07-23接受日期:2024-09-05出版日期:2025-12-08发布日期:2025-01-17 -
通讯作者:廖红兵,博士,教授,广西医科大学口腔医学院/附属口腔医院口腔修复科,广西口腔颌面修复与重建研究重点实验室,广西壮族自治区南宁市 530021 -
作者简介:吴子炜,男,1998 年生,湖北省洪湖市人,汉族,广西医科大学在读硕士,主要从事骨替代材料与组织工程学研究。 -
基金资助:国家自然科学基金资助项目(82160192),项目负责人:廖红兵
Application of poly(lactic-co-glycolic acid) copolymer in stomatology
Wu Ziwei, Luo Yicai, Wei Yinge, Liao Hongbing
- Department of Oral Prosthodontics, School of Stomatology/Affiliated Stomatological Hospital, Guangxi Medical University, Guangxi Key Laboratory of Oral and Maxillofacial Prosthodontics and Reconstruction, Nanning 530021, Guangxi Zhuang Autonomous Region, China
-
Received:2024-07-23Accepted:2024-09-05Online:2025-12-08Published:2025-01-17 -
Contact:Liao Hongbing, MD, Professor, Department of Oral Prosthodontics, School of Stomatology/Affiliated Stomatological Hospital, Guangxi Medical University, Guangxi Key Laboratory of Oral and Maxillofacial Prosthodontics and Reconstruction, Nanning 530021, Guangxi Zhuang Autonomous Region, China -
About author:Wu Ziwei, Master candidate, Department of Oral Prosthodontics, School of Stomatology/Affiliated Stomatological Hospital, Guangxi Medical University, Guangxi Key Laboratory of Oral and Maxillofacial Prosthodontics and Reconstruction, Nanning 530021, Guangxi Zhuang Autonomous Region, China -
Supported by:National Natural Science Foundation of China, No. 82160192 (to LHB)
摘要:
文题释义:
聚乳酸-羟基乙酸共聚物:由乳酸和羟基乙酸2种单体按不同比例随机聚合而成,这种人工合成的聚合物具有生物相容性、降解性、无毒、成囊和成膜等优点,被广泛应用于生物医用工程、制药及现代化工业领域。口腔医学:主要涉及口腔软硬组织,包括对组织生长发育及病变机制的研究,是口腔及颌面部疾病诊断、治疗、预防等方面的科学。
背景:聚乳酸-羟基乙酸共聚物因良好的生物安全性、生物降解性及优越的机械性能已成为口腔医学领域的研究热点。
目的:综述聚乳酸-羟基乙酸共聚物在口腔医学领域的研究进展。方法:利用计算机检索中国知网和PubMed数据库中的相关文献,中文检索词为“聚乳酸-羟基乙酸,PLGA,口腔缺损,组织工程”;英文检索词为“PLGA,Polylactic acid-hydroxyacetic acid copolymer,Poly (lactic-co-glycolic acid) copolymer,dent*,regeneration,caries,periodontal,pulp,implant,alveolar bone”。通过阅读文题和摘要进行初步筛选,排除与文章主题不相关的文献,根据纳入标准和排除标准最终纳入119篇文献进行综述。
结果与结论:聚乳酸-羟基乙酸共聚物在口腔医学领域的应用范围正迅速拓展,逐步取代传统的治疗药物和修复材料。聚乳酸-羟基乙酸共聚物纳米粒子/微球能够负载多种疏水和亲水性活性物质,在龋病预防、根管消毒和盖髓治疗方面具有优秀的递送能力。聚乳酸-羟基乙酸共聚物可以屏障膜和药物载体的形式用于牙周组织再生。聚乳酸-羟基乙酸共聚物用于种植体表面改性,不仅增强了植体表面的抗菌能力,而且改善了植体表面的生物惰性。单纯聚乳酸-羟基乙酸共聚物支架治疗骨缺损的效果有限,需要结合3D打印、各种生物活性成分、无机材料等提高支架性能。聚乳酸-羟基乙酸共聚物联合干细胞可以提高神经损伤修复效果,符合临床需要。基于聚乳酸-羟基乙酸共聚物在口腔医学领域的巨大潜力,未来有望根据口腔组织工程的不同需求生产出针对不同疾病具有特定功能的修复材料。
https://orcid.org/0009-0009-0796-1617 (吴子炜)
中国组织工程研究杂志出版内容重点:生物材料;骨生物材料;口腔生物材料;纳米材料;缓释材料;材料相容性;组织工程
中图分类号:
引用本文
吴子炜, 罗诒财, 韦银格, 廖红兵. 聚乳酸-羟基乙酸共聚物在口腔医学领域的应用[J]. 中国组织工程研究, 2025, 29(34): 7393-7404.
Wu Ziwei, Luo Yicai, Wei Yinge, Liao Hongbing. Application of poly(lactic-co-glycolic acid) copolymer in stomatology[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7393-7404.
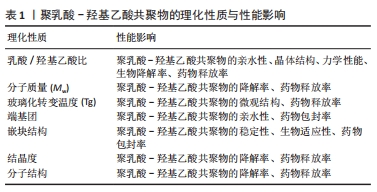
聚乳酸-羟基乙酸共聚物保留了2种单体的一些性质,如乳酸的刚性、疏水性和缓慢降解性,以及羟基乙酸的延伸性、相对较小的疏水性和较快的降解性[17],因此乳酸/羟基乙酸比值显著影响了聚乳酸-羟基乙酸共聚物的亲水性、晶体结构、力学性能和生物降解率[18]。乳酸含量较高的聚乳酸-羟基乙酸共聚物亲水性较低,往往吸收更少的水,因此呈现更长的降解时间;羟基乙酸含量较高的聚乳酸-羟基乙酸共聚物亲水性较高,具有较高的水合速率、更快的降解速度[19]。聚乳酸-羟基乙酸共聚物的分子质量(Mw)常常被认为是影响其降解和药物释放的重要因素之一。聚乳酸-羟基乙酸共聚物的分子质量越高,其聚合物链越长、降解需要的时间越长,从而导致更慢的释放速率[2]。通常来讲,分子质量较高的聚乳酸-羟基乙酸共聚物具有更慢的降解速率和药物释放动力学,因为它需要更多的时间来水解成可溶性低聚物[20]。玻璃化转变温度是指聚合物从玻璃态转变为高弹态所对应的温度范围[21]。聚乳酸-羟基乙酸共聚物的玻璃化转变温度通常在40-60 ℃之间,这决定了聚乳酸-羟基乙酸共聚物的微观结构和药物释放[22]。同样的,聚乳酸-羟基乙酸共聚物的玻璃化转变温度会随乳酸/羟基乙酸比值和分子质量而变化,主要表现为玻璃化转变温度随着乳酸含量和聚乳酸-羟基乙酸共聚物分子质量的降低而降低,因此,聚乳酸-羟基乙酸共聚物的玻璃化转变温度对其降解速率的影响是间接的[23]。
聚乳酸-羟基乙酸共聚物的自由端基可以影响聚合物的性质。聚乳酸-羟基乙酸共聚物的自由端基分为端羟基、端羧基和端酯基。一般来说,端羟基和端酯基的聚乳酸-羟基乙酸共聚物疏水性更强,降解速度更慢[24]。端羧基可以催化酯键的水解,从而产生更多的酸性基团和建立一个加速聚合物降解的自动催化循环,因此,端羧基的聚乳酸-羟基乙酸共聚物亲水性更强,降解周期更快[25]。此外,端基团还能显著影响药物包封率和聚合物的承载能力。
聚乳酸-羟基乙酸共聚物可以与其他共聚物进行嵌段聚合,从而对聚乳酸-羟基乙酸共聚物进行改性,更好地满足其载体功能的需要。聚乙二醇和聚乳酸-羟基乙酸共聚物的二嵌段共聚物(聚乳酸-羟基乙酸共聚物-聚乙二醇)和三嵌段共聚物(聚乳酸-羟基乙酸共聚物-聚乙二醇-聚乳酸-羟基乙酸共聚物)是提高聚乳酸-羟基乙酸共聚物稳定性和生物适应性的常用方法[18]。聚乙二醇具有活性羟基末端,能够与大量活性药物分子偶联,与聚乳酸-羟基乙酸共聚物聚合后可以在其表面形成聚乙二醇层,进而增加聚乳酸-羟基乙酸共聚物的稳定性[26]。事实上,聚乙二醇化已被证明可以改善封装在聚乳酸-羟基乙酸共聚物复合材料中药物的药代动力学特性[27];用生物相容性亲水聚合物(聚乙二醇或壳聚糖)涂层的聚乳酸-羟基乙酸共聚物可以提高材料的稳定性和循环时间,同时减少毒性[28]。
聚乳酸-羟基乙酸共聚物的结晶度指其分子链在空间中有序排列的程度,聚乳酸-羟基乙酸共聚物的结晶度会影响其物理性质、降解速率和药物释放性能。聚乳酸-羟基乙酸共聚物的结晶度范围从完全非晶态到完全结晶态,由其嵌块结构和摩尔比决定[29]。聚乳酸-羟基乙酸共聚物的单体种类是根据乳酸的类型来决定的,乳酸具有不同的旋光性(D型、L型及DL型3种类型),导致聚乳酸-羟基乙酸共聚物具有不同的异构体[30]。聚(D、L-乳酸)和羟基乙酸合成的聚乳酸-羟基乙酸共聚物具有非晶结构,而聚(L-乳酸)和羟基乙酸合成的聚乳酸-羟基乙酸共聚物共聚物具有结晶性质。此外,值得注意的是,小于70%羟基乙酸合成的聚乳酸-羟基乙酸共聚物也是无定形的[31]。
聚乳酸-羟基乙酸共聚物的分子结构可分为线形或支链形(星形、多臂形、环形等),这些结构上的差异会影响聚乳酸-羟基乙酸共聚物的降解性,同时也会影响药物的缓释效应[32]。线性聚乳酸-羟基乙酸共聚物往往由开环聚合获得,支链形聚乳酸-羟基乙酸共聚物可以采取多种方式获得,例如开环聚合通过添加一个含有多羟基(葡萄糖)的引发剂作为核心,激活羟基来修饰合成星形聚乳酸-羟基乙酸共聚物。不同结构聚乳酸-羟基乙酸共聚物在相同溶剂中的分子构象会因空间位阻的不同而有所差异,线形聚乳酸-羟基乙酸共聚物的空间位阻比支链形聚乳酸-羟基乙酸共聚物小,从而引起聚乳酸-羟基乙酸共聚物微球制剂的差异[33]。
综上所述,聚乳酸-羟基乙酸共聚物的合成及理化性质不仅决定了其在生物医学领域的应用范围,还影响着其在临床应用中的表现和效果。通过调控这些理化性质,聚乳酸-羟基乙酸共聚物能够更好地满足口腔医学的需求。文章总结了聚乳酸-羟基乙酸共聚物相关的理化性质,见表1。
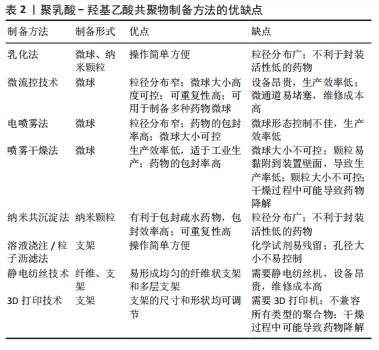
2.2 聚乳酸-羟基乙酸共聚物的制备方法 聚乳酸-羟基乙酸共聚物可以溶于多种有机溶剂,如氯仿、乙酮、乙酸乙酯和四氢呋喃[29],因此,可以通过各种制备方法得到多样化结构的聚乳酸-羟基乙酸共聚物生物材料。聚乳酸-羟基乙酸共聚物在口腔医学领域的二级结构包括微球、纳米颗粒、屏障膜、支架和纤维等。
微球和纳米颗粒的小尺寸结构提高了封装分子的生物利用度,其可观的表面积-体积比便于表面修饰;更重要的是,它们可以更有效地穿透细胞以实现靶向给药[29,34]。因此,聚乳酸-羟基乙酸共聚物微球和聚乳酸-羟基乙酸共聚物纳米颗粒在药物递送系统中起着重要作用。乳化法是制备聚乳酸-羟基乙酸共聚物微球和聚乳酸-羟基乙酸共聚物纳米颗粒最常见的方法,常用于温度敏感药物的封装,操作过程简单,可以在一定程度上控制颗粒的大小[35]。乳化法可分为单乳化方法(O/W)和双重乳化方法(W1/O/W2、S/O/W和 W/O/O),前者常用于封装疏水药物分子,后者常用于封装亲水药物分子[36]。微流控技术是生产高度控制和均匀微球的新兴技术,该方法得到的聚乳酸-羟基乙酸共聚物微球具有粒径分布集中和重复性高等优点[37]。电喷雾法是将聚乳酸-羟基乙酸共聚物溶液注入注射器,然后在注射器口和收集基板之间施加一定的电压,喷射出的溶液在注射器口形成一个特殊的“泰勒锥”,形成液滴并进一步雾化成粒子,最后以相反的电荷进入收集基底[38]。喷雾干燥法是一种将药物和聚合物溶液、悬浮液或乳状液雾化并注入热空气中的技术,通过将聚乳酸-羟基乙酸共聚物溶液进行喷洒,使其在热干燥介质中(空气、惰性气体如氮气)转化为颗粒[39]。纳米共沉淀法也称为相分离法,通过将聚乳酸-羟基乙酸共聚物溶解在有机相中,然后将稳定剂注射到水相中,沉淀后得到聚乳酸-羟基乙酸共聚物纳米颗粒,该方法通常用于封装亲脂性药物。
支架因一定的机械强度和空间结构常常用于骨组织工程。溶液浇注/粒子沥滤法是一种传统的制造聚乳酸-羟基乙酸共聚物支架的方法,先将聚乳酸-羟基乙酸共聚物溶解在有机溶剂中,然后加入制孔剂混匀,待有机溶剂挥发后将混合物在模具内成型,最后成型的制品在无酶水中浸泡,待干燥后得到聚乳酸-羟基乙酸共聚物支架[40]。静电纺丝技术可以作为电喷雾法的一个特例,可以制备不同尺寸纳米结构的均匀纤维,常用于制造聚乳酸-羟基乙酸共聚物纳米纤维,也可以制造聚乳酸-羟基乙酸共聚物支架,该方法需要在高压下将静电纺丝溶液向外喷射,在收集板上形成纳米纤维或支架[41]。3D打印技术因可以定制个性化的支架,是制作聚乳酸-羟基乙酸共聚物个性化支架的热门技术,其内容包括但不限于熔融沉积、挤压打印和低温打印[42]。
综上所述,不同制备方法可以获得不同形态的聚乳酸-羟基乙酸共聚物生物材料,满足口腔不同部位的治疗需求。文章总结了聚乳酸-羟基乙酸共聚物的制备方法,见表2。
2.3 聚乳酸-羟基乙酸共聚物在口腔疾病中的的应用
2.3.1 龋病防治 龋齿是一种慢性、进行性和破坏性的疾病,主要由细菌感染引起。研究表明,龋齿已成为世界范围内一个严重的公共卫生问题,因此,预防龋齿具有重要的意义[43]。预防龋病的关键在于破坏致龋菌形成的生物膜(如变形链球菌),由于口腔中的动态环境,传统的抗菌药物难以渗透和吸附在致龋生物膜[44]。聚乳酸-羟基乙酸共聚物作为一种优秀的药物载体,可以有效地递送药物,被用于预防龋病。
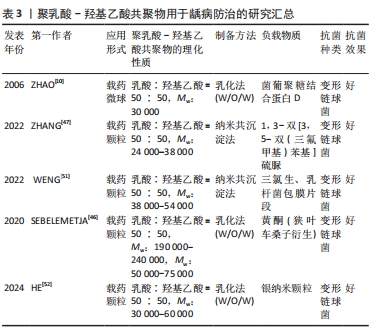
许多研究表明,鼻内免疫可以在相对容易使用的情况下用较低剂量的抗原最大限度地提高保护性唾液IgA抗体水平[45]。然而,可溶性蛋白抗原通常不能通过这种方式获得令人满意的免疫应答。在此基础上,ZHAO等[10]在大肠杆菌中克隆并表达了葡聚糖结合蛋白D,然后将其包封在壳聚糖包覆的聚乳酸-羟基乙酸共聚物微球中,用于大鼠的龋齿治疗,结果表明,将葡聚糖结合蛋白D装入壳聚糖包被的聚乳酸-羟基乙酸共聚物微球中可提高其鼻内免疫后的免疫原性和保护性免疫能力,证明了壳聚糖修饰聚乳酸-羟基乙酸共聚物微球作为鼻内免疫疫苗传递系统的潜力。
除了通过接种疫苗的方式来防治龋病,研究人员也尝试寻找新的抗龋药物。SEBELEMETJA等[46]从狭叶车桑子提取黄酮,用聚乳酸-羟基乙酸共聚物纳米粒子包裹负载以稳定其活性,结果显示该纳米颗粒保留了黄酮已知的抗变形链球菌能力,并在生理pH值和致龋pH值下长时间缓慢释放黄酮,表明用黄酮稳定的聚乳酸-羟基乙酸共聚物纳米粒子具有抗变形链球菌的潜力。ZHANG等[47]将1,3-双[3,5-双(三氟甲基)苯基]硫脲作为一种抗致龋菌的潜在药物,将其封装在聚乳酸-羟基乙酸共聚物纳米颗粒中,探讨了其作为防龋剂的潜力,结果显示,载药聚乳酸-羟基乙酸共聚物纳米颗粒具有令人满意的表面形貌、粒径、粒径分布和稳定性,对人口腔鳞癌细胞的细胞毒性可以忽略不计;相比于正常pH值,该纳米颗粒在酸性环境(pH=4.5)的药物累积释放率更高,显著抑制了变形链球菌生物膜的生长和乳酸的累积。
近年来,一种新型的纳米颗粒传递系统(即细胞膜包覆纳米颗粒)引起了广泛关注。细胞膜包覆纳米颗粒是通过将源细胞外膜直接覆盖在合成纳米颗粒的表面来制备的,具有模拟细胞的特性[48]。三氯生自1997年获得FDA批准以来,被广泛应用于牙膏和漱口水等口腔护理产品中,研究表明三氯生对变异链球菌有明显的抑制作用[49]。最近的研究认为,乳酸菌菌株即使被热杀死也能抑制变形链球菌在口腔中的定植和生物膜的形成[50]。在此基础上,WENG等[51]首先制备了乳杆菌包膜片段/三氯生@聚乳酸-羟基乙酸共聚物纳米颗粒,该纳米颗粒可以黏附于变形链球菌并整合到变形链球菌的生物膜中,干扰变形链球菌生物膜的形成;此外,该纳米颗粒在体外显著抑制了变形链球菌生物膜的活性、生物量和毒力基因的表达,这些结果显示,该纳米颗粒在体内对龋病的进展具有长期抑制作用。HE等[52]开发了一种“智能粒子”用于靶向致龋生物膜,将银纳米颗粒(BPEI-AgNPs)封装在单宁酸?铁(Ⅲ)修饰的聚乳酸-羟基乙酸共聚物纳米颗粒中,使其可以在pH=4.0(致龋pH值)下响应释放Ag+,起到破坏致龋生物膜的作用;抗菌实验结果表明,“智能粒子”在酸性条件下可以显著降低变形链球菌的生物膜体积和活力,细胞毒性实验显示“智能粒子”对人牙龈成纤维细胞没有细胞毒性,这些结果表明“智能粒子”在治疗龋齿方面具有巨大的潜力。
聚乳酸-羟基乙酸共聚物在龋病防治中的进展研究总结,见表3。
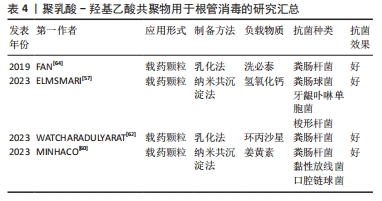
2.3.2 根管消毒 根管系统具有复杂的结构,如根管侧支、管间吻合、根尖分歧、根尖分叉和副根管等,这些复杂结构的存在使得细菌微生物的清除具有高度挑战性,即使用机械清创和化学清洗也很难从根管系统中完全清除细菌[53]。根管消毒的目的是消除根管内病原体和受感染组织,防止进一步感染,促进根尖周炎的愈合[54]。
氢氧化钙是临床上最常用的根管消毒剂,其抗菌作用主要是由于它产生的碱性环境,分离的羟基离子会作用于细菌细胞壁,通过蛋白质变性导致细胞质膜损伤及DNA损伤[55]。氢氧化钙的持续释放有利于维持周围环境的高碱性pH值,进而维持羟基离子的抗菌活性[56],因此,一个合适的药物载体应该允许氢氧化钙缓慢而稳定地释放钙离子和羟基离子,从而最终决定氢氧化钙的临床表现。在此基础上,ELMSMAR等[57]设计了负载氢氧化钙的聚乳酸-羟基乙酸共聚物纳米颗粒,该纳米颗粒的粒径平均大小在200 nm以下,抗菌实验结果显示,载药颗粒对牙龈卟啉单胞菌和粪肠球菌的最低抑菌浓度为10 μg/mL,对梭形杆菌的最低抑菌浓度为5 μg/mL,虽然在琼脂扩散实验中没有观察到抑制区域,但载药颗粒显著降低了细菌的代谢活性,从而保持了活性化合物的有效性。综合结果表明,与单独使用氢氧化钙相比,负载氢氧化钙的聚乳酸-羟基乙酸共聚物纳米颗粒对根管消毒具有更好的抗菌效果。
抗菌光动力疗法被认为是一种很有前途的替代传统根管消毒策略,这种方法包括光敏剂和光源(通常是低强度激光器)的结合,通过产生活性氧来抑制广谱微生物[58]。姜黄素是一种从姜科植物的根茎中提取的天然酚类抗氧化剂,由于姜黄素在300-500 nm之间存在长吸收峰,并且对细胞培养模型和动物模型没有毒性作用,因此被作为一种有效的光动力治疗的光敏剂[59]。MIVHACO等[60]采用纳米共沉淀法合成和表征一种新的含姜黄素的聚乳酸-羟基乙酸共聚物纳米颗粒,然后结合蓝色发光二极管评价了其作为光敏剂的抗菌活性,研究结果显示:在蓝光激活下,纳米颗粒对粪肠杆菌菌、黏性放线菌和口腔链球菌均表现优秀抗菌性能;细胞实验结果显示,接受纳米颗粒+姜黄素325 μg/mL和纳米颗粒+姜黄素200 μg/mL治疗组细胞数量显著增加,结果证明这种含有姜黄素的新型聚合物纳米颗粒可能是疗根管感染一种有效手段。
环丙沙星是一种针对粪肠杆菌的广谱抗生素,通常是作为根管药物的抗生素组分,然而,亲水性环丙沙星的高浓度酸性可能会抑制根尖乳头干细胞的增殖和分化,进而导致根管治疗的失败[61];此外,治疗药物的迅速降解可导致抗生素浓度降低到有效剂量以下。因此,开发一种新的局部抗生素给药系统对于延长药物的活性和提高细胞相容性至关重要。WATCHARADULYARAT等[62]制备了负载环丙沙星的聚乙二醇-聚乳酸-羟基乙酸共聚物纳米颗粒,该纳米颗粒对粪肠杆菌的最低抑菌浓度为(1.07±0.19) μg/mL,最低杀菌浓度为(2.83±1.04) μg/mL,细胞毒性实验结果表明,负载环丙沙星的质量浓度在5-100 μg/mL范围内,对根尖乳头干细胞的毒性较低。因此,聚乳酸-羟基乙酸共聚物纳米颗粒作为环丙沙星的载体可以延长其有效性、增加其亲脂性,进而增强杀菌作用。
洗必泰是一种公认的杀菌剂,可针对多种革兰阳性和革兰阴性微生物,包括粪肠杆菌。在牙科治疗中洗必泰通常以溶液或凝胶的形式使用,这样的应用形式只能作用于根管的牙本质表面,不能浸润到牙本质小管中[63]。FAN等[64]制备了一种装载和释放洗必泰以及钙和磷的聚乳酸-羟基乙酸共聚物亚微米颗粒,以改进洗必泰的应用,抗菌实验结果显示,该亚微米颗粒在所有浓度下对粪肠杆菌均有显著的杀菌作用;细胞毒性实验结果显示,该亚微米颗粒对MC3T3-E1细胞增殖没有抑制作用;显微硬度实验结果显示,该亚微米颗粒治疗后的牙本质切片显微硬度明显增加。因此,装载洗必泰以及钙和磷的聚乳酸-羟基乙酸共聚物颗粒可作为一种新的有效牙科治疗根管消毒剂。
聚乳酸-羟基乙酸共聚物在根管治疗中应用的进展研究总结,见表4。
2.3.3 盖髓治疗 针对牙髓感染,传统的治疗方法是根管治疗,通过清除坏死的牙髓组织,用合成生物材料充填根管来控制炎症,这种治疗方法往往很少有牙髓组织的再生,使得治疗后的牙齿失去了牙髓固有的自然生物防御,进而影响牙齿的存活率[65]。目前,牙髓组织再生被认为是治疗牙髓感染的新方向。
人牙髓干细胞由GRONTHOS等[66]首次分离出来,具有多向分化的潜能,一直在组织工程的种子细胞中扮演着重要的角色。然而,人牙髓干细胞通常来自成年患者阻生牙,并且拔牙过程延迟了干细胞的及时分离,从而导致人牙髓干细胞来源有限。人脱落乳牙干细胞以微创的方式从脱落乳牙中获得,表现出非免疫原性和多向分化潜能,可以作为牙髓组织工程的种子细胞,但人脱落乳牙干细胞在坏死髓腔微环境中促进牙髓再生的能力仍然有限[67]。
辛伐他汀是一种用于治疗高脂血症的小分子药物,已被证明具有多种功能,包括抗炎和抗氧化潜能,可促进矿化组织沉积[68]。有研究发现,负载辛伐他汀的壳聚糖支架增加了牙髓细胞的趋化性和再生潜能[69]。YUAN等[70]研究了一种辛伐他汀功能化甲基丙烯酸明胶低温凝胶微球,用以装载人脱落乳牙干细胞,以完善人脱落乳牙干细胞的生物学行为,结果显示,负载辛伐他汀的聚乳酸-羟基乙酸共聚物纳米颗粒可以维持辛伐他汀的持续释放,增加甲基丙烯酸明胶冷冻凝胶微球的机械性能,该微球增强了人脱落乳牙干细胞的牙源性分化和血管生成潜能,促进人脐静脉内皮细胞的迁移和血管生成,展现了其在促进血管化组织再生方面的潜力;将微球注射到清洁过的离体牙根段中并植入裸鼠皮下,显示该微球可以诱导富含血管的牙髓样组织再生,表明这种可注射的纳米级生物活性试剂系统可以适用于牙髓再生的临床应用。
临床上传统的盖髓剂材料是氢氧化钙和三氧化物矿物骨料,两者对修复性牙本质的形成有良好的促进作用[71],但它们也存在抗炎作用较差、处理困难、牙齿变色等缺点。阿司匹林是一种广泛应用于临床的非类固醇抗炎药物,有研究表明,阿司匹林可降低牙髓干细胞中炎症因子的表达水平,说明阿司匹林可在控制牙髓炎症反应中发挥有效作用[72]。非晶型磷酸钙已被证明具有良好的生物降解性和生物活性,并且无细胞毒性,非晶型磷酸钙支架已被证明可以持续释放钙离子和磷离子,因其优异的生物活性、高细胞黏附性和可调节的生物降解率而被广泛应用于生物医学领域[73]。YAN等[74]设计了一种结构为3层的复合膜,与牙髓接触的最下层为含有高浓度非晶型磷酸钙的阿司匹林/聚乳酸-羟基乙酸共聚物膜,中间层为含有低浓度的阿司匹林和非晶型磷酸的阿司匹林/非晶型磷酸钙/聚左旋乳酸膜,外层为聚乳酸-羟基乙酸共聚物膜,最下层的阿司匹林/聚乳酸-羟基乙酸共聚物膜可快速释放阿司匹林,控制牙髓炎症;中间层的阿司匹林/非晶型磷酸钙/聚左旋乳酸膜可长期释放钙磷离子和阿司匹林,促进修复性牙本质的形成;外层的聚乳酸-羟基乙酸共聚物膜层可以改善复合膜的表面亲水性,有利于提高盖髓材料的密封性能,表明该复合膜可以诱导牙髓炎模型中修复性牙本质的形成。DAGHRERY等[75]先将β-环糊精包裹地塞米松以改善其水溶性,然后用聚乳酸-羟基乙酸共聚物纤维丝负载,该纤维在体外可以持续稳定地释放地塞米松,促进人乳牙干细胞的成骨分化,显示其在牙髓再生中的潜力。
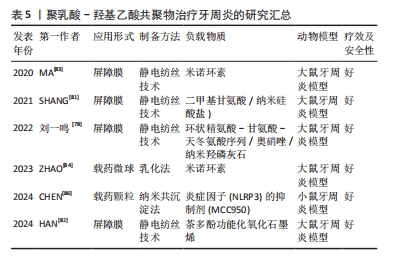
2.3.4 牙周再生 牙周炎是一种慢性炎症疾病,以牙龈出血、附着丧失、牙周袋形成和牙槽骨吸收为特征[76]。牙周组织一旦受到损伤,几乎没有自我修复的倾向,因此,需要有效的干预来重建丢失或损伤的组织,其中最常用的方法是引导组织再生[77]。聚乳酸-羟基乙酸共聚物因良好的生物降解性和生物相容性及易于操作的能力,成为引导组织再生膜的候选材料。
刘一鸣等[78]设计了一种具有抑制纤维化及抑菌作用的功能化复合静电纺丝纤维膜,该膜有3层结构,内层为负载环状精氨酸-甘氨酸-天冬氨酸序列、奥硝唑及纳米羟磷灰石的功能层,外层为抑制纤维化作用的功能层,中间层具有支撑作用及隔离不同功能层作用的致密膜层,体外实验结果显示该复合膜的内层可支持 MC3T3-E1细胞增殖,外层可抑制 L929细胞增殖,负载奥硝唑的复合膜可抑制牙龈卟啉单胞菌的增殖;体内实验结果显示,与临床常用的胶原口腔修复膜相比,负载奥硝唑的复合膜修复大鼠颌骨缺损的愈合效果最好。成骨-血管生成的耦合过程在牙周组织再生中起着至关重要的作用。二甲基甘氨酸可以间接激活低氧诱导因子1信号通路从而上调血管生成因子的表达,如血管内皮生长因子和基质衍生因子[79]。纳米硅酸盐由生物活性硅酸盐纳米片组成,是一种合成的二维盘状纳米材料,其在促进干细胞成骨分化方面引起了研究人员的关注[80]。SHANG等[81]将二甲基甘氨酸和纳米硅酸盐结合到聚乳酸-羟基乙酸共聚物纤维中,开发一种用于牙周组织再生的双功能结构,其在体外可以促进牙周韧带干细胞向成骨分化与血管生成标志物的表达,在体内可以促进大鼠牙周缺损模型的修复,表明该纤维膜可以增强和协调成骨-血管生成,并有可能在牙周组织工程中成为一种有效的支架。HAN等[82]以茶多酚功能化氧化石墨烯、聚乳酸-羟基乙酸共聚物和聚己内酯为原料,设计了一种新型纤维膜,该纤维膜在体外可以通过磷脂酰肌醇3-激酶/蛋白激酶B和核因子κB信号通路来抑制巨噬细胞(RAW 264.7)向M1型极化,促进牙周韧带干细胞的成骨分化,在体内修复大鼠牙周缺损。MA等[83]制备了负载米诺环素的聚乳酸-羟基乙酸共聚物纤维膜,该纤维膜体外药物持续释可达40 d,促进成骨细胞的增殖、刺激牙周炎模型的骨再生。
抗菌药物和生长因子已被证明有利于牙周组织再生,聚乳酸-羟基乙酸共聚物作为一种理想的药物传递系统,允许负载物质以预期的速率和浓度释放。ZHAO等[84]设计负载米诺环素的聚乳酸-羟基乙酸共聚物微球用于治疗牙周炎,探讨了聚乳酸-羟基乙酸共聚物理化性质对负载和释放米诺环素的影响,结果发现聚乳酸-羟基乙酸共聚物的羧基可以与米诺环素的羟基、碳基和酰胺基形成氢键,提高了两者的化学结合,通过调整聚乳酸-羟基乙酸共聚物的乳酸和羟基乙酸比、分子质量和端基团可以调控米诺环素的释放行为,实现牙周炎的程序性治疗。慢性牙周炎的晚期糖基化终末产物(AGEs)在病变部位的大量积累会加重牙周组织损伤,巨噬细胞在其中起着重要的调控作用[85]。CHEN等[86]设计了新型聚乳酸-羟基乙酸共聚物纳米载药颗粒(GLU@MCC),通过葡萄糖转运体实现主动靶向晚期糖基化终末产物诱导的M1巨噬细胞,释放出炎症因子(NLRP3)的抑制剂(MCC950),体外实验证实GLU@MCC的作用机制可能与在M1巨噬细胞中抑制NLRP3炎症因子密切相关;体内实验表明GLU@MCC可修复小鼠的牙周组织损伤,抑制细胞凋亡。综上所述,GLU@MCC可通过M1巨噬细胞中的葡萄糖转运体作为靶点,抑制炎症因子(NLRP3)的表达,治疗慢性牙周炎。
聚乳酸-羟基乙酸共聚物在牙周治疗中应用的进展研究总结,见表5。
2.3.5 种植体表面改性 种植体相关感染是种植术后的严重并发症。钛及其合金作为应用最广泛的种植体材料,由于其表面存在生物惰性且缺乏抗菌性能,容易引起的种植体相关感染[87-88]。因此,预防种植体感染和提高其骨诱导能力是一个重要的目标[89]。聚乳酸-羟基乙酸共聚物具有良好的生物相容性,可以负载一些抗菌生物材料在种植体表面形成抗菌涂层,赋予钛基质抗菌特性。
KAZEK-K?SIK等[90]首先对钛合金表面进行阳极氧化获得有利于细胞黏附的多孔氧化层,然后采用浸涂技术形成包裹阿莫西林层的聚乳酸-羟基乙酸共聚物功能性涂层,抗菌实验结果显示,聚乳酸-羟基乙酸共聚物涂层1 h内释放的药物浓度足以抑制金黄色葡萄球菌和表皮葡萄球菌的生长;细胞毒性实验结果显示,聚乳酸-羟基乙酸共聚物涂层不影响MG-63成骨细胞的黏附和增殖。随着纳米技术的发展,银纳米颗粒逐渐成为骨组织工程研究的重点,其抗菌能力可以通过破坏细胞膜来杀死细菌;此外有研究发现,银纳米颗粒还具有一定的促成骨能力[91]。尽管具有一定的抗菌潜力,但高浓度的银纳米颗粒可能抑制成骨细胞的增殖,产生不必要的不良反应,并且银纳米颗粒有聚集和形成大团簇的趋势,需要聚合物作为颗粒的稳定体系促进了银纳米颗粒的均匀分布[92]。ZENG等[93]制备了包裹银纳米颗粒的聚乳酸-羟基乙酸共聚物涂层钛,与未涂层的钛种植体相比,涂层钛可以显著抑制金黄色葡萄球菌和铜绿假单胞菌的黏附,具有优越的抗菌和骨诱导活性。由于抗菌物质只存在于植体表面,在使用过程中涂层的抗菌活性可能会逐渐下降或完全丧失,因此,还需要添加具有抗菌活性的合金元素。GENG等[94]将银纳米颗粒与钛-铜合金结合,构建了银离子与铜离子协同作用的新型抗菌种植体,该植入体具有释放银离子和铜离子的能力,在体外可以促进MC3T3-E1细胞的成骨分化,在体内可以抑制大鼠股骨骨髓炎,促进骨缺损的修复。YANG等[95]将银纳米颗粒和Fe3O4 成功结合,并和聚乳酸-羟基乙酸共聚物形成复合涂层,该涂层不仅具有超顺磁性,还可以与含永磁铁的种植体紧密结合,并且表现出与银纳米颗粒几乎相同的抗菌活性。以上的涂层技术采用剂量递增法来延长银纳米颗粒的作用时间,在不影响生物相容性的前提下提高种植体的抗菌活性。
2.3.6 颌骨修复 颌骨的感染来源很多,包括牙源性、血源性、腺源性、创伤性和医源性感染,感染途径的多样性以及部位的特殊性给颌骨缺损的修复带来极大挑战。聚乳酸-羟基乙酸共聚物因优秀的性能已被认为是自体骨移植、同种异体移植或异种移植的有效替代品,并被设计成支架、屏障膜或植骨材料用于颌骨修复[96]。
上颌窦提升术常用于上颌窦底过低、牙槽嵴高度不能满足种植体长度要求的情况。聚乳酸-羟基乙酸共聚物/羟基磷灰石作为一种新型的植骨材料,具有令人满意的临床疗效[97]。PORTELLI等[98]比较了去蛋白牛骨和聚乳酸-羟基乙酸共聚物/羟基磷灰石应用于上颌窦底提升术的效果,锥形束CT分析结果显示,与去蛋白牛骨相比,聚乳酸-羟基乙酸共聚物/羟基磷灰石植入后可以在7个月内被完全重吸收,并且新形成活骨的百分比高于去蛋白牛骨组。RODRIGUEZ等[99]探讨聚乳酸-羟基乙酸共聚物/羟基磷灰石联合自体骨膜在上颌窦提升术的植骨效果,实验分为3组:A组(聚乳酸-羟基乙酸共聚物/羟基磷灰石+骨膜),B组(单独聚乳酸-羟基乙酸共聚物/羟基磷灰石),C组(单独Bio-Oss?),研究结果显示A组的骨再生明显快于B组和C组,并且A组术后4个月后锥形束CT显示出现骨结合,植体2年后继续维持。FLICHY-FERNáNDEZ等[100]将聚乳酸-羟基乙酸共聚物包裹的双相磷酸钙颗粒用于上颌窦提升术,发现聚乳酸-羟基乙酸共聚物包裹的双相磷酸钙颗粒与未包裹的磷酸钙颗粒组织形态测量结果无差异;但免疫组化结果显示,聚乳酸-羟基乙酸共聚物包裹的颗粒被更多的成骨细胞(msi1阳性)定植,并被更多的CD34阳性血管结构穿透,说明其与植骨区的微血管化程度有关。
大部分患者在种植手术过程中常因牙槽骨骨量不足需进行骨增量手术,引导骨再生是实现骨增量最常用的技术。PETPOSRI等[101]采用3D打印技术制备了不同乳酸/羟基乙酸比值的聚乳酸-羟基乙酸共聚物膜,发现聚乳酸-羟基乙酸共聚物(70∶30)膜具有更好的机械强度,对成纤维细胞和成骨细胞增殖的支持能力明显优于聚乳酸-羟基乙酸共聚物(90∶10)膜;与市售的胶原蛋白膜相比,聚乳酸-羟基乙酸共聚物(70∶30)膜对大鼠颅骨缺损的修复效果更好。TANG等[102]以聚乳酸-羟基乙酸共聚物为基质制备了一种具有致密和多孔双重结构的双层膜,阻隔软组织的致密层与锌颗粒结合,以促进骨再生的多孔层与镁和锌颗粒共同结合,结果显示,与单独聚乳酸-羟基乙酸共聚物膜相比,镁/锌/聚乳酸-羟基乙酸共聚物膜的机械强度更高,并且由于离子释放的连续性,使膜具有优异的成骨和抗菌活性,这为组织引导再生治疗提供了新的设计思路。
单独的聚乳酸-羟基乙酸共聚物支架在应用上往往存在一定的局限,因此常结合具有骨转导和骨诱导功能的无机材料,其中最常用的是羟基磷灰石和磷酸三钙,它们是骨组织中的天然成分,具有较强的骨传导性和骨诱导[103]。3D打印技术可以轻松重建所需的三维模型,并准确生成与缺损区域相匹配的骨移植材料,常用于制备聚乳酸-羟基乙酸共聚物支架。QIAN等[104]将含有磷酸钙骨水泥和硅灰(WS)的浆料渗透到3D打印聚乳酸-羟基乙酸共聚物支架中,结果表明该支架在加热后恢复了磷酸钙骨水泥的塑性、增加了磷酸钙骨水泥在前期的灵活性,并于降解时在原位产生三维互联的大孔;硅灰石有助于改善小鼠骨髓间充质干细胞的附着、增殖和成骨分化;体内实验结果表明该支架可促进快速血管生成和骨形成。虽然有机-无机复合材料改善了聚乳酸-羟基乙酸共聚物支架的成骨性能,但尚未解决机械强度不足的问题。因此,LAI等[105]制备了一种新型多孔聚乳酸-羟基乙酸共聚物/磷酸三钙/镁支架,力学实验结果显示镁的加入提高了支架的机械性能,与聚乳酸-羟基乙酸共聚物/磷酸三钙支架相比,聚乳酸-羟基乙酸共聚物/磷酸三钙/镁支架的抗压强度与弹性模量均升高;兔股骨缺损模型也证明了聚乳酸-羟基乙酸共聚物/磷酸三钙/镁支架的优异成骨性能。
单纯使用材料提高生物相容性的方法存在局限性,为了进一步提高聚乳酸-羟基乙酸共聚物支架的生物相容性,可以在支架上加载生长因子或药物。DENG等[106]构建含有重组骨形态发生蛋白2的壳聚糖纳米缓释载体,通过3D低温打印技术将重组骨形态发生蛋白2壳聚糖纳米缓释载体负载到聚乳酸-羟基乙酸共聚物/纳米羟基磷灰石支架上,体外实验证实,重组骨形态发生蛋白2的壳聚糖纳米缓释载体释放细胞因子的剂量-时间效应关系可以满足组织工程骨的成骨要求;体内实验证明,负载壳聚糖纳米缓释载体的支架可修复兔下颌骨缺损。近年来,除了生长因子和药物外,干细胞在骨组织工程中也有着广阔的应用前景。PROBST等[107]将脂肪干细胞加载到磷酸三钙/聚乳酸-羟基乙酸共聚物支架上,通过猪下颌骨缺损模型发现,磷酸三钙/聚乳酸-羟基乙酸共聚物支架在有细胞或无细胞的情况下修复骨缺损的潜力,而负载脂肪干细胞的磷酸三钙/聚乳酸-羟基乙酸共聚物支架获得了更高的新骨体积和骨密度,证明了负载活细胞的3D打印支架更满足临床需要。
聚乳酸-羟基乙酸共聚物在颌骨修复应用的进展研究总结,见表6。

2.3.7 神经损伤治疗 由于口腔颌面部的特殊位置和解剖结构,往往出现神经损伤,其中面神经损伤是最常见的。在临床治疗中,远距离神经缺损一般定义为大于10 mm的神经缺损,当这种情况发生时,简单的神经缝合可能会导致手术困难,并由于过度张力而抑制轴突再生。而自体或异体神经移植也存在供体稀少、损伤大、免疫排斥等问题,导致远距离面神经损伤治疗效果不理想,严重影响患者的生活质量[108]。组织工程的发展可以为神经再生提供一个治疗的新方向。
聚乳酸-羟基乙酸共聚物由于生物安全性和降解性可以避免二次手术取出,被用于修复神经损伤。SASAKI 等[109]以大鼠牙髓干细胞作为种子细胞、聚乳酸-羟基乙酸共聚物制备的神经管作为当神经修复引导器,用于修复大鼠面神经的损伤,结果显示:与对照组相比,含有牙髓干细胞的聚乳酸-羟基乙酸共聚物神经管移植5 d后可见神经修复,移植2个月后免疫染色显示神经中存在阳性轴突,说明存在神经再生。近年来,研究人员致力于设计功能更为全面的合成导管来满足神经再生的需要。血管内皮生长因子是一种高度保守的二聚体糖蛋白,在刺激血管生成的同时,不仅保护受损神经元,还促进神经再生[110]。有研究表明,血管内皮生长因子可以诱导内皮细胞建立血管系统,从而引导施万细胞定向迁移,加速神经轴突的生长[111]。XU等[112]通过慢病毒转染生成过表达血管内皮生长因子的大鼠牙髓干细胞,促进干细胞向神经向分化,然后使用新型层粘连蛋白涂层和纱线包裹的聚乳酸-羟基乙酸共聚物神经导管来连接面神经缺损,并将过表达血管内皮生长因子的干细胞注射到神经导管中,结果表明,过表达血管内皮生长因子的大鼠牙髓干细胞在髓鞘发育、轴突生长和功能恢复方面具有一定的优势,过表达血管内皮生长因子的大鼠牙髓干细胞结合聚乳酸-羟基乙酸共聚物神经导管可以加速大鼠远程面神经损伤的修复过程,为神经组织工程提供了一个新的思路。

| [1] HAN S, YANG H, NI X, et al. Programmed release of vascular endothelial growth factor and exosome from injectable chitosan nanofibrous microsphere-based PLGA-PEG-PLGA hydrogel for enhanced bone regeneration. Int J Biol Macromol. 2023;253(Pt 1):126721. [2] LIM YW, TAN WS, HO KL, et al. Challenges and Complications of Poly(lactic-co-glycolic acid)-Based Long-Acting Drug Product Development. Pharmaceutics. 2022;14(3):614. [3] 张城,颜东,常罡,等.PLGA分子特性对其微球性质影响的研究进展[J].中国医药工业杂志,2023,54(9):1294-1301. [4] JIN S, XIA X, HUANG J, et al. Recent advances in PLGA-based biomaterials for bone tissue regeneration. Acta Biomater. 2021;127: 56-79. [5] ARAUJO-PIRES AC, MENDES VC, FERREIRA-JUNIOR O, et al. Investigation of a Novel PLGA/CaP Scaffold in the Healing of Tooth Extraction Sockets to Alveolar Bone Preservation in Humans. Clin Implant Dent Relat Res. 2016;18(3):559-570. [6] DAVIES JE, MATTA R, MENDES VC, et al. Development, characterization and clinical use of a biodegradable composite scaffold for bone engineering in oro-maxillo-facial surgery. Organogenesis. 2010;6(3):161-166. [7] SOUSA FF, LUZARDO-ALVAREZ A, PÉREZ-ESTÉVÉZ A, et al. Development of a novel AMX-loaded PLGA/zein microsphere for root canal disinfection. Biomed Mater. 2010;5(5):055008. [8] PAGONIS TC, CHEN J, FONTANA CR, et al. Nanoparticle-based endodontic antimicrobial photodynamic therapy. J Endod. 2010;36(2):322-328. [9] KARFELD-SULZER LS, GHAYOR C, SIEGENTHALER B, et al. Comparative study of NMP-preloaded and dip-loaded membranes for guided bone regeneration of rabbit cranial defects. J Tissue Eng Regen Med. 2017;11(2):425-433. [10] ZHAO H, WU B, WU H, et al. Protective immunity in rats by intranasal immunization with Streptococcus mutans glucan-binding protein D encapsulated into chitosan-coated poly(lactic-co-glycolic acid) microspheres. Biotechnol Lett. 2006;28(16):1299-1304. [11] GALA-GARCIA A, TEIXEIRA KI, WYKROTA FH, et al. Bioceramic/poly (glycolic)-poly (lactic acid) composite induces mineralized barrier after direct capping of rat tooth pulp tissue. Braz Oral Res. 2010;24(1):8-14. [12] MUNDARGI RC, SRIRANGARAJAN S, AGNIHOTRI SA, et al. Development and evaluation of novel biodegradable microspheres based on poly(d,l-lactide-co-glycolide) and poly(epsilon-caprolactone) for controlled delivery of doxycycline in the treatment of human periodontal pocket: in vitro and in vivo studies. J Control Release. 2007;119(1):59-68. [13] VAN OIRSCHOT B, MIKOS AG, LIU Q, et al. Fast Degradable Calcium Phosphate Cement for Maxillofacial Bone Regeneration. Tissue Eng Part A. 2023;29(5-6): 161-171. [14] GHANDFOROUSHAN P, HANAEE J, AGHAZADEH Z, et al. Novel nanocomposite scaffold based on gelatin/PLGA-PEG-PLGA hydrogels embedded with TGF-β1 for chondrogenic differentiation of human dental pulp stem cells in vitro. Int J Biol Macromol. 2022;201:270-287. [15] 张晓宇,陈琪,杨兴,等.聚乳酸-羟基乙酸共聚物微球在骨组织工程中的应用[J].中国组织工程研究,2023,27(30):4896-1903. [16] CHOI SY, KIM WJ, YU SJ, et al. Engineering the xylose-catabolizing Dahms pathway for production of poly(d-lactate-co-glycolate) and poly(d-lactate-co-glycolate-co-d-2-hydroxybutyrate) in Escherichia coli. Microb Biotechnol. 2017;10(6):1353-1364. [17] ESSA D, KONDIAH PPD, CHOONARA YE, et al. The Design of Poly(lactide-co-glycolide) Nanocarriers for Medical Applications. Front Bioeng Biotechnol. 2020;8:48. [18] MAKADIA HK, SIEGEL SJ. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers (Basel). 2011;3(3):1377-1397. [19] 卢仁培,邹志晨,赵丰年,等.聚乳酸-羟基乙酸共聚物复合支架在骨缺损修复再生中的作用与应用 [J].中国组织工程研究,2022,26(28):4525-4531. [20] PATEL RB, CARLSON AN, SOLORIO L, et al. Characterization of formulation parameters affecting low molecular weight drug release from in situ forming drug delivery systems. J Biomed Mater Res A. 2010;94(2):476-484. [21] PARK K, OTTE A, SHARIFI F, et al. Potential Roles of the Glass Transition Temperature of PLGA Microparticles in Drug Release Kinetics. Mol Pharm. 2021;18(1):18-32. [22] HUA Y, WANG Z, WANG D, et al. Key Factor Study for Generic Long-Acting PLGA Microspheres Based on a Reverse Engineering of Vivitrol(®). Molecules. 2021;26(5):1247. [23] LIU G, MCENNIS K. Glass Transition Temperature of PLGA Particles and the Influence on Drug Delivery Applications. Polymers (Basel). 2022;14(5):993. [24] LI X, WEI Y, WEN K, et al. Novel insights on the encapsulation mechanism of PLGA terminal groups on ropivacaine. Eur J Pharm Biopharm. 2021;160:143-151. [25] WANG J, HELDER L, SHAO J, et al. Encapsulation and release of doxycycline from electrospray-generated PLGA microspheres: Effect of polymer end groups. Int J Pharm. 2019;564:1-9. [26] SUN S, CUI Y, YUAN B, et al. Drug delivery systems based on polyethylene glycol hydrogels for enhanced bone regeneration. Front Bioeng Biotechnol. 2023;11: 1117647. [27] KHALIL NM, DO NASCIMENTO TC, CASA DM, et al. Pharmacokinetics of curcumin-loaded PLGA and PLGA-PEG blend nanoparticles after oral administration in rats. Colloids Surf B Biointerfaces. 2013;101:353-360. [28] NOORI KOOPAEI M, KHOSHAYAND MR, MOSTAFAVI SH, et al. Docetaxel Loaded PEG-PLGA Nanoparticles: Optimized Drug Loading, In-vitro Cytotoxicity and In-vivo Antitumor Effect. Iran J Pharm Res. 2014;13(3):819-833. [29] HASSAN M, ABDELNABI HA, MOHSIN S. Harnessing the Potential of PLGA Nanoparticles for Enhanced Bone Regeneration. Pharmaceutics. 2024;16(2):273. [30] 陈泽宇,付烨,张茜,等.PLGA的降解行为及应用研究进展[J].中国塑料, 2024,38(1):92-99. [31] DINARVAND R, SEPEHRI N, MANOOCHEHRI S, et al. Polylactide-co-glycolide nanoparticles for controlled delivery of anticancer agents. Int J Nanomedicine. 2011;6:877-895. [32] HUA Y, SU Y, ZHANG H, et al. Poly(lactic-co-glycolic acid) microsphere production based on quality by design: a review. Drug Deliv. 2021;28(1):1342-1355. [33] HADAR J, SKIDMORE S, GARNER J, et al. Characterization of branched poly (lactide-co-glycolide) polymers used in injectable, long-acting formulations. J Control Release. 2019;304:75-89. [34] MATSUMOTO A, MURAKAMI M. Harmless and ecologically acceptable fabrication of long-acting injectable microspheres. Drug Discov Ther. 2023;17(3):170-176. [35] HAN FY, THURECHT KJ, WHITTAKER AK, et al. Bioerodable PLGA-Based Microparticles for Producing Sustained-Release Drug Formulations and Strategies for Improving Drug Loading. Front Pharmacol. 2016;7:185. [36] RAMAZANI F, CHEN W, VAN NOSTRUM CF, et al. Strategies for encapsulation of small hydrophilic and amphiphilic drugs in PLGA microspheres: State-of-the-art and challenges. Int J Pharm. 2016;499(1-2):358-367. [37] CABALLERO AGUILAR LM, DUCHI S, ONOFRILLO C, et al. Formation of alginate microspheres prepared by optimized microfluidics parameters for high encapsulation of bioactive molecules. J Colloid Interface Sci. 2021;587:240-251. [38] MALIK SA, NG WH, BOWEN J, et al. Electrospray synthesis and properties of hierarchically structured PLGA TIPS microspheres for use as controlled release technologies. J Colloid Interface Sci. 2016;467:220-229. [39] WEI H, LI W, CHEN H, et al. Simultaneous Diels-Alder click reaction and starch hydrogel microsphere production via spray drying. Carbohydr Polym. 2020;241: 116351. [40] HUANG Y, REN J, REN T, et al. Bone marrow stromal cells cultured on poly (lactide-co-glycolide)/nano-hydroxyapatite composites with chemical immobilization of Arg-Gly-Asp peptide and preliminary bone regeneration of mandibular defect thereof. J Biomed Mater Res A. 2010;95(4):993-1003. [41] YAO J, LIU Z, MA W, et al. Three-Dimensional Coating of SF/PLGA Coaxial Nanofiber Membranes on Surfaces of Calcium Phosphate Cement for Enhanced Bone Regeneration. ACS Biomater Sci Eng. 2020;6(5):2970-2984. [42] BABILOTTE J, MARTIN B, GUDURIC V, et al. Development and characterization of a PLGA-HA composite material to fabricate 3D-printed scaffolds for bone tissue engineering. Mater Sci Eng C Mater Biol Appl. 2021;118:111334. [43] ZHOU X, XU X, LI J, et al. Oral health in China: from vision to action. Int J Oral Sci. 2018;10(1):1. [44] SIMS KR, LIU Y, HWANG G, et al. Enhanced design and formulation of nanoparticles for anti-biofilm drug delivery. Nanoscale. 2018;11(1):219-236. [45] LEHNER T, CHALLACOMBE SJ. Letter: Immunisation against dental caries. Br Med J. 1975;4(5999):760. [46] SEBELEMETJA M, MOENO S, PATEL M. Anti-acidogenic, anti-biofilm and slow release properties of Dodonaea viscosa var. angustifolia flavone stabilized polymeric nanoparticles. Arch Oral Biol. 2020;109:104586. [47] ZHANG M, LIAO Y, TONG X, et al. Novel urea derivative-loaded PLGA nanoparticles to inhibit caries-associated Streptococcus mutans. RSC Adv. 2022;12(7):4072-4080. [48] NARAIN A, ASAWA S, CHHABRIA V, et al. Cell membrane coated nanoparticles: next-generation therapeutics. Nanomedicine (Lond). 2017;12(21):2677-2692. [49] DE SOUZA ARAÚJO IJ, DE PAULA AB, BRUSCHI ALONSO RC, et al. A novel Triclosan Methacrylate-based composite reduces the virulence of Streptococcus mutans biofilm. PLoS One. 2018;13(4):e0195244. [50] WASFI R, ABD EL-RAHMAN OA, ZAFER MM, et al. Probiotic Lactobacillus sp. inhibit growth, biofilm formation and gene expression of caries-inducing Streptococcus mutans. J Cell Mol Med. 2018;22(3):1972-1983. [51] WENG L, WU L, GUO R, et al. Lactobacillus cell envelope-coated nanoparticles for antibiotic delivery against cariogenic biofilm and dental caries. J Nanobiotechnology. 2022;20(1):356. [52] HE Y, BRIGHT R, VASILEV K, et al. Development of “Intelligent particles” for the treatment of dental caries. Eur J Pharm Biopharm. 2024;202:114374. [53] SIQUEIRA JF JR, RÔÇAS IN. Present status and future directions: Microbiology of endodontic infections. Int Endod J. 2022;55 Suppl 3:512-530. [54] NAIR PN. Pathogenesis of apical periodontitis and the causes of endodontic failures. Crit Rev Oral Biol Med. 2004;15(6):348-381. [55] ROIG-SORIANO X, SOUTO EB, ELMSMARI F, et al. Nanoparticles in Endodontics Disinfection: State of the Art. Pharmaceutics. 2022;14(7):1519. [56] MOHAMMADI Z, DUMMER PM. Properties and applications of calcium hydroxide in endodontics and dental traumatology. Int Endod J. 2011;44(8):697-730. [57] ELMSMARI F, DELGADO LM, DURAN-SINDREU F, et al. Novel strategies enhancing endodontic disinfection: Antibacterial biodegradable calcium hydroxide nanoparticles in an ex vivo model. Int J Pharm. 2023;648:123627. [58] TRINDADE AC, DE FIGUEIREDO JA, STEIER L, et al. Photodynamic therapy in endodontics: a literature review. Photomed Laser Surg. 2015;33(3) 175-182. [59] HAUKVIK T, BRUZELL E, KRISTENSEN S, et al. Photokilling of bacteria by curcumin in selected polyethylene glycol 400 (PEG 400) preparations. Studies on curcumin and curcuminoids, XLI. Pharmazie. 2010;65(8):600-606. [60] MINHACO V, MAQUERA HUACHO PM, MANCIM IMBRIANI MJ, et al. Improving antimicrobial activity against endodontic biofilm after exposure to blue light-activated novel curcumin nanoparticle. Photodiagnosis Photodyn Ther. 2023; 42:103322. [61] ARAÚJO PRS, SILVA LB, NETO A, et al. Pulp Revascularization: A Literature Review. Open Dent J. 2017;10:48-56. [62] WATCHARADULYARAT N, RATTANATAYAROM M, RUANGSAWASDI N, et al. PEG-PLGA nanoparticles for encapsulating ciprofloxacin. Sci Rep. 2023;13(1):266. [63] HASHEMINIA S, FARHAD AR, SAATCHI M, et al. Synergistic antibacterial activity of chlorhexidine and hydrogen peroxide against Enterococcus faecalis. J Oral Sci. 2013;55(4):275-280. [64] FAN W, LI Y, LIU D, et al. PLGA submicron particles containing chlorhexidine, calcium and phosphorus inhibit Enterococcus faecalis infection and improve the microhardness of dentin. J Mater Sci Mater Med. 2019;30(2):17. [65] SCHMALZ G, WIDBILLER M, GALLER KM. Clinical Perspectives of Pulp Regeneration. J Endod. 2020;46(9s):S161-s74. [66] GRONTHOS S, MANKANI M, BRAHIM J, et al. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97(25):13625-13630. [67] KO CS, CHEN JH, SU WT. Stem Cells from Human Exfoliated Deciduous Teeth: A Concise Review. Curr Stem Cell Res Ther. 2020;15(1):61-76. [68] XU J, LIU X, CHEN J, et al. Simvastatin enhances bone marrow stromal cell differentiation into endothelial cells via notch signaling pathway. Am J Physiol Cell Physiol. 2009;296(3):C535-543. [69] SOARES DG, ANOVAZZI G, BORDINI EAF, et al. Biological Analysis of Simvastatin-releasing Chitosan Scaffold as a Cell-free System for Pulp-dentin Regeneration. J Endod. 2018;44(6):971-976.e1. [70] YUAN X, YUAN Z, WANG Y, et al. Vascularized pulp regeneration via injecting simvastatin functionalized GelMA cryogel microspheres loaded with stem cells from human exfoliated deciduous teeth. Mater Today Bio. 2022;13:100209. [71] SONG M, YU B, KIM S, et al. Clinical and Molecular Perspectives of Reparative Dentin Formation: Lessons Learned from Pulp-Capping Materials and the Emerging Roles of Calcium. Dent Clin North Am. 2017;61(1):93-110. [72] LIU Y, CHEN C, LIU S, et al. Acetylsalicylic acid treatment improves differentiation and immunomodulation of SHED. J Dent Res. 2015; 94(1):209-218. [73] SU Y, ZHANG B, SUN R, et al. PLGA-based biodegradable microspheres in drug delivery: recent advances in research and application. Drug Deliv. 2021; 28(1):1397-1418. [74] YAN W, YANG F, LIU Z, et al. Anti-Inflammatory and Mineralization Effects of an ASP/PLGA-ASP/ACP/PLLA-PLGA Composite Membrane as a Dental Pulp Capping Agent. J Funct Biomater, 2022;13(3):106. [75] DAGHRERY A, AYTAC Z, DUBEY N, et al. Electrospinning of dexamethasone/cyclodextrin inclusion complex polymer fibers for dental pulp therapy. Colloids Surf B Biointerfaces. 2020;191:111011. [76] SUSIN C, WIKESJÖ UM. Regenerative periodontal therapy: 30 years of lessons learned and unlearned. Periodontol 2000. 2013;62(1):232-242. [77] SAM G, PILLAI BR. Evolution of Barrier Membranes in Periodontal Regeneration-”Are the third Generation Membranes really here?”. J Clin Diagn Res. 2014; 8(12):Ze14-17. [78] 刘一鸣,赵云,韩梅,等.功能化聚乳酸-羟基乙酸共聚物基骨组织再生诱导膜的制备及其在大鼠颌骨缺损重建中的应用[J].华西口腔医学杂志, 2022,40(5):522-531. [79] QI X, LIU Y, DING Z Y, et al. Synergistic effects of dimethyloxallyl glycine and recombinant human bone morphogenetic protein-2 on repair of critical-sized bone defects in rats. Sci Rep. 2017;7:42820. [80] GAHARWAR AK, CROSS LM, PEAK CW, et al. 2D Nanoclay for Biomedical Applications: Regenerative Medicine, Therapeutic Delivery, and Additive Manufacturing. Adv Mater. 2019;31(23):e1900332. [81] SHANG L, LIU Z, MA B, et al. Dimethyloxallyl glycine/nanosilicates-loaded osteogenic/angiogenic difunctional fibrous structure for functional periodontal tissue regeneration. Bioact Mater. 2021;6(4):1175-1188. [82] HAN X, WANG F, MA Y, et al. TPG-functionalized PLGA/PCL nanofiber membrane facilitates periodontal tissue regeneration by modulating macrophages polarization via suppressing PI3K/AKT and NF-κB signaling pathways. Mater Today Bio. 2024;26:101036. [83] MA Y, SONG J, ALMASSRI HNS, et al. Minocycline-loaded PLGA electrospun membrane prevents alveolar bone loss in experimental peridontitis. Drug Deliv. 2020;27(1):151-160. [84] ZHAO J, WEI Y, XIONG J, et al. Antibacterial-Anti-Inflammatory-Bone Restoration Procedure Achieved by MIN-Loaded PLGA Microsphere for Efficient Treatment of Periodontitis. AAPS PharmSciTech. 2023; 24(3):74. [85] FRETWURST T, GARAICOA-PAZMINO C, NELSON K, et al. Characterization of macrophages infiltrating peri-implantitis lesions. Clin Oral Implants Res. 2020; 31(3):274-281. [86] CHEN Q, ZHAO Y, XIE C, et al. Therapeutic Effect of a Novel M1 Macrophage-Targeted Nanodrug in Chronic Periodontitis Mice. Mol Pharm. 2024;21(4):1677-1690. [87] WANG B, LAN J, QIAO H, et al. Porous surface with fusion peptides embedded in strontium titanate nanotubes elevates osteogenic and antibacterial activity of additively manufactured titanium alloy. Colloids Surf B Biointerfaces. 2023; 224:113188. [88] LI X, XU M, GENG Z, et al. Novel pH-Responsive CaO(2)@ZIF-67-HA-ADH Coating That Efficiently Enhances the Antimicrobial, Osteogenic, and Angiogenic Properties of Titanium Implants. ACS Appl Mater Interfaces. 2023;15(36):42965-42980. [89] WU H, CHEN X, KONG L, et al. Mechanical and Biological Properties of Titanium and Its Alloys for Oral Implant with Preparation Techniques: A Review. Materials (Basel). 2023;16(21):6860. [90] KAZEK-KĘSIK A, NOSOL A, PŁONKA J, et al. PLGA-amoxicillin-loaded layer formed on anodized Ti alloy as a hybrid material for dental implant applications. Mater Sci Eng C Mater Biol Appl. 2019;94:998-1008. [91] ZHANG R, LEE P, LUI VC, et al. Silver nanoparticles promote osteogenesis of mesenchymal stem cells and improve bone fracture healing in osteogenesis mechanism mouse model. Nanomedicine. 2015;11(8):1949-1959. [92] CAO H, ZHANG W, MENG F, et al. Osteogenesis Catalyzed by Titanium-Supported Silver Nanoparticles. ACS Appl Mater Interfaces. 2017;9(6):5149-5157. [93] ZENG X, XIONG S, ZHUO S, et al. Nanosilver/poly (dl-lactic-co-glycolic acid) on titanium implant surfaces for the enhancement of antibacterial properties and osteoinductivity. Int J Nanomedicine. 2019;14:1849-1863. [94] GENG Z, DONG R, LI X, et al. Study on the Antibacterial Activity and Bone Inductivity of Nanosilver/PLGA-Coated TI-CU Implants. Int J Nanomedicine. 2024;19:6427-6447. [95] YANG Y, REN S, ZHANG X, et al. Safety and efficacy of PLGA(Ag-Fe(3)O(4))-coated dental implants in inhibiting bacteria adherence and osteogenic inducement under a magnetic field. Int J Nanomedicine. 2018;13:3751-3762. [96] PIAO ZG, KIM JS, SON JS, et al. Osteogenic evaluation of collagen membrane containing drug-loaded polymeric microparticles in a rat calvarial defect model. Tissue Eng Part A. 2014;20(23-24):3322-3331. [97] RANCITELLI D, BORGONOVO AE, CICCIÙ M, et al. Maxillary Sinus Septa and Anatomic Correlation With the Schneiderian Membrane. J Craniofac Surg. 2015; 26(4):1394-1398. [98] PORTELLI M, CICCIÙ M, LAURITANO F, et al. Histomorphometric Evaluation of Two Different Bone Substitutes in Sinus Floor Augmentation Procedures. J Craniofac Surg. 2017. doi: 10.1097/SCS.0000000000003572. [99] RODRIGUEZ YBR, D’AQUINO R, GRAZIANO A, et al. Autologous Periosteum-Derived Micrografts and PLGA/HA Enhance the Bone Formation in Sinus Lift Augmentation. Front Cell Dev Biol. 2017;5:87. [100] FLICHY-FERNÁNDEZ AJ, BLAYA-TÁRRAGA JA, O’VALLE F, et al. Sinus floor elevation using particulate PLGA-coated biphasic calcium phosphate bone graft substitutes: A prospective histological and radiological study. Clin Implant Dent Relat Res. 2019;21(5):895-902. [101] PETPOSRI S, THUAKSUBAN N, BURANADHAM S, et al. Physical Characteristics and Biocompatibility of 3D-Printed Polylactic-Co-Glycolic Acid Membranes Used for Guided Bone Regeneration. J Funct Biomater. 2023;14(5):275. [102] TANG H, QI C, BAI Y, et al. Incorporation of Magnesium and Zinc Metallic Particles in PLGA Bi-layered Membranes with Sequential Ion Release for Guided Bone Regeneration. ACS Biomater Sci Eng. 2023;9(6):3239-3252. [103] WON JY, PARK CY, BAE JH, et al. Evaluation of 3D printed PCL/PLGA/β-TCP versus collagen membranes for guided bone regeneration in a beagle implant model. Biomed Mater. 2016;11(5):055013. [104] QIAN G, FAN P, HE F, et al. Novel Strategy to Accelerate Bone Regeneration of Calcium Phosphate Cement by Incorporating 3D Plotted Poly(lactic-co-glycolic acid) Network and Bioactive Wollastonite. Adv Healthc Mater. 2019; 8(9):e1801325. [105] LAI Y, LI Y, CAO H, et al. Osteogenic magnesium incorporated into PLGA/TCP porous scaffold by 3D printing for repairing challenging bone defect. Biomaterials. 2019;197:207-219. [106] DENG N, SUN J, LI Y, et al. Experimental study of rhBMP-2 chitosan nano-sustained release carrier-loaded PLGA/nHA scaffolds to construct mandibular tissue-engineered bone. Arch Oral Biol. 2019;102:16-25. [107] PROBST FA, FLIEFEL R, BURIAN E, et al. Bone regeneration of minipig mandibular defect by adipose derived mesenchymal stem cells seeded tri-calcium phosphate- poly(D,L-lactide-co-glycolide) scaffolds. Sci Rep. 2020;10(1):2062. [108] SULLIVAN R, DAILEY T, DUNCAN K, et al. Peripheral Nerve Injury: Stem Cell Therapy and Peripheral Nerve Transfer. Int J Mol Sci. 2016;17(12):2101. [109] SASAKI R, AOKI S, YAMATO M, et al. PLGA artificial nerve conduits with dental pulp cells promote facial nerve regeneration. J Tissue Eng Regen Med. 2011;5(10): 823-830. [110] THEIS V, THEISS C. VEGF - A Stimulus for Neuronal Development and Regeneration in the CNS and PNS. Curr Protein Pept Sci. 2018; 19(6):589-597. [111] MATHOT F, SHIN AY, VAN WIJNEN AJ. Targeted stimulation of MSCs in peripheral nerve repair. Gene. 2019;710:17-23. [112] XU W, XU X, YAO L, et al. VEGFA-modified DPSCs combined with LC-YE-PLGA NGCs promote facial nerve injury repair in rats. Heliyon. 2023;9(4):e14626. [113] AL-AHMAD A, WIEDMANN-AL-AHMAD M, CARVALHO C, et al. Bacterial and Candida albicans adhesion on rapid prototyping-produced 3D-scaffolds manufactured as bone replacement materials. J Biomed Mater Res A. 2008; 87(4):933-943. [114] MOLLY L, VANDROMME H, QUIRYNEN M, et al. Bone formation following implantation of bone biomaterials into extraction sites. J Periodontol. 2008;79(6): 1108-1115. [115] MINENNA L, HERRERO F, SANZ M, et al. Adjunctive effect of a polylactide/polyglycolide copolymer in the treatment of deep periodontal intra-osseous defects: a randomized clinical trial. J Clin Periodontol. 2005;32(5):456-461. [116] KUBOTA K, OCHI R, TUGE Y, et al. [Experimental study of periodontal tissue regeneration using biodegradable membranes]. Nihon Shishubyo Gakkai Kaishi. 1989;31(3):870-881. [117] BROWN A, ZAKY S, RAY H JR, et al. Porous magnesium/PLGA composite scaffolds for enhanced bone regeneration following tooth extraction. Acta Biomater. 2015;11:543-553. [118] SERINO G, BIANCU S, IEZZI G, et al. Ridge preservation following tooth extraction using a polylactide and polyglycolide sponge as space filler: a clinical and histological study in humans. Clin Oral Implants Res. 2003;14(5):651-658. [119] CHANG CJ, HSU SH. The effect of high outflow permeability in asymmetric poly(dl-lactic acid-co-glycolic acid) conduits for peripheral nerve regeneration. Biomaterials. 2006;27(7):1035-1042. |
| [1] | 王奇飒, 卢雨征, 韩秀峰, 赵文玲, 石海涛, 徐 哲. 3D打印甲基丙烯酰化透明质酸/脱细胞皮肤水凝胶支架的细胞相容性[J]. 中国组织工程研究, 2026, 30(8): 1912-1920. |
| [2] | 许 畅, 姜明珠, 刘 鑫, 闫伟军. 种植支抗联合转矩辅弓压低前牙的三维有限元分析[J]. 中国组织工程研究, 2026, 30(8): 1971-1978. |
| [3] | 郑旭颖, 胡洪成, 许礼兵, 韩建民, 邸 萍. 不同载荷形式和内连接形状下两段式粘接固位氧化锆种植体的应力大小和分布[J]. 中国组织工程研究, 2026, 30(8): 1979-1987. |
| [4] | 王菘芃, 刘玉三, 于焕英, 高晓丽, 徐英江, 张晓明, 刘 敏. 沸石基咪唑盐框架8纳米材料的活性氧双向调控:从肿瘤治疗、抗菌到细胞保护[J]. 中国组织工程研究, 2026, 30(8): 2033-2013. |
| [5] | 杨学涛, 朱梦菡, 张宸熙, 孙一民, 叶 玲. 抗氧化纳米材料在口腔中的应用和不足[J]. 中国组织工程研究, 2026, 30(8): 2044-2053. |
| [6] | 陈豪杰, 王 黛, 沈 山. 种植体周围炎中的免疫炎症微环境机制[J]. 中国组织工程研究, 2026, 30(8): 2054-2062. |
| [7] | 杨琼琼, 刘 玮. 氧化锆与钛种植体的性能及临床效果对比[J]. 中国组织工程研究, 2026, 30(8): 2063-2071. |
| [8] | 刘 洋, 刘东辉, 徐 磊, 展 旭, 孙昊博, 康 凯. 刺激响应型可注射水凝胶在心肌梗死精准化治疗中的作用与趋势[J]. 中国组织工程研究, 2026, 30(8): 2072-2080. |
| [9] | 刘大为, 崔颖颖, 王方辉, 王子轩, 陈宇涵, 李友瑞, 张荣和. 表没食子儿茶素没食子酸酯介导活性氧双向调控及在纳米材料中的应用[J]. 中国组织工程研究, 2026, 30(8): 2101-2112. |
| [10] | 赖 渝, 陈跃平, 章晓云. 生物活性材料治疗骨感染的研究热点与前沿趋势[J]. 中国组织工程研究, 2026, 30(8): 2132-2144. |
| [11] | 吴妍廷, 李 宇, 廖金凤. 氧化镁纳米粒调控成骨与血管生成相关基因表达促进骨缺损愈合[J]. 中国组织工程研究, 2026, 30(8): 1885-1895. |
| [12] | 刘新月, 李春年, 李一卓, 徐世芳. 口腔牙槽骨缺损的再生修复[J]. 中国组织工程研究, 2026, 30(5): 1247-1259. |
| [13] | 徐沈聪, 方紫菲, 季明意, 徐诚睿, 李彬红, 曹佳雨, 徐俊峰. 外斜线Onlay植骨在上前牙骨缺损种植修复中的应用[J]. 中国组织工程研究, 2026, 30(4): 841-848. |
| [14] | 王明琦, 冯诗雅, 韩银河, 于朋鑫, 郭丽娜, 贾子萱, 王秀丽. 神经化肠黏膜组织工程模型的构建及体外评价[J]. 中国组织工程研究, 2026, 30(4): 892-900. |
| [15] | 余诗宇, 俞苏桐, 徐 杨, 镇祥燕, 韩凤选. 组织工程治疗策略在口腔黏膜下纤维化中的研究与应用进展[J]. 中国组织工程研究, 2026, 30(4): 936-948. |
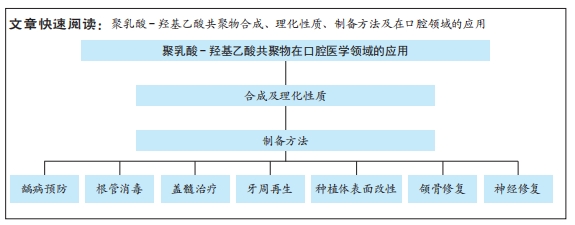
聚乳酸-羟基乙酸共聚物(PLGA)作为一种人工合成的高分子材料具有多种优秀性质,被广泛应用于生物医学领域[1]。自1989年起,聚乳酸-羟基乙酸共聚物已经被美国食品和药品管理局(FDA)和欧洲药品管理局(EMA)批准用于生物医学领域[2]。截至2022年10月,中国上市的9款长效注射聚乳酸-羟基乙酸共聚物微球制剂已广泛用于相关疾病的治疗[3]。基于聚乳酸-羟基乙酸共聚物的外科产品,如可吸收缝线(Coated VICRYL®)、骨螺钉和骨移植体(OsteoScafTM)等[4-5],被广泛应用于外科手术。传统药物(如氢氧化钙、三氧矿物聚合物、洗必泰等)在口腔疾病治疗方面存在一定的局限性。近年来,随着加工技术和制造工艺的发展,基于聚乳酸-羟基乙酸共聚物的生物材料在口腔医学领域取得了重大进展。目前,各种应用形式的聚乳酸-羟基乙酸共聚物修复材料已被开发出来,如支架[6]、微球[7]、纳米粒子[8]、膜等[9](图1),应用于各种口腔疾病的治疗,例如龋病[10]、牙髓病[11]、牙周病[12]、颌骨缺损等[13]。
 聚乳酸-羟基乙酸共聚物已被广泛应用于组织工程和药物递送等,有关聚乳酸-羟基乙酸共聚物在口腔疾病治疗中的研究多局限于颌面骨缺损修复,在口腔其他部位的应用研究较少,并且缺乏对聚乳酸-羟基乙酸共聚物独特性能的分析。文章就聚乳酸-羟基乙酸共聚物的优秀性能与其在口腔疾病中的应用展开研究,介绍聚乳酸-羟基乙酸共聚物的合成、理化性质、制备方法以及在口腔领域的应用,简要讨论聚乳酸-羟基乙酸共聚物的新趋势和未来方向,为进一步推动聚乳酸-羟基乙酸共聚物在口腔领域的发展提供参考。
中国组织工程研究杂志出版内容重点:生物材料;骨生物材料;口腔生物材料;纳米材料;缓释材料;材料相容性;组织工程
聚乳酸-羟基乙酸共聚物已被广泛应用于组织工程和药物递送等,有关聚乳酸-羟基乙酸共聚物在口腔疾病治疗中的研究多局限于颌面骨缺损修复,在口腔其他部位的应用研究较少,并且缺乏对聚乳酸-羟基乙酸共聚物独特性能的分析。文章就聚乳酸-羟基乙酸共聚物的优秀性能与其在口腔疾病中的应用展开研究,介绍聚乳酸-羟基乙酸共聚物的合成、理化性质、制备方法以及在口腔领域的应用,简要讨论聚乳酸-羟基乙酸共聚物的新趋势和未来方向,为进一步推动聚乳酸-羟基乙酸共聚物在口腔领域的发展提供参考。
中国组织工程研究杂志出版内容重点:生物材料;骨生物材料;口腔生物材料;纳米材料;缓释材料;材料相容性;组织工程
1.1.1 检索人及检索时间 由第一作者在2024年5月进行检索。
1.1.2 文献检索时限 2000年1月至2024年5月,同时纳入少数远期经典及特别相关文献。
1.1.3 检索数据库 中国知网、PubMed数据库。
1.1.4 检索词 中文检索词为“聚乳酸-羟基乙酸,PLGA,口腔缺损,组织工程”;英文检索词为“PLGA,Polylactic acid-hydroxyacetic acid copolymer,Poly (lactic-co-glycolic acid) copolymer,dent*,regeneration,caries,periodontal,pulp,implant,alveolar bone”。
1.1.5 检索文献类型 综述、研究原著及著作。
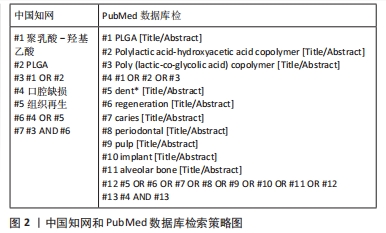
1.1.6 检索策略 中国知网、PubMed数据库检索策略,见图2。
1.2 纳入及排除标准
1.2.1 纳入标准 ①与聚乳酸-羟基乙酸共聚物有关的文献;②与口腔组织工程有关的文献;同一领域相关研究选择近年发表的文献。
1.2.2 排除标准 ①不符合该文主题的文献或重复研究;②质量较低、文献资料来源不够权威的文献;③无法查看全文的文献。
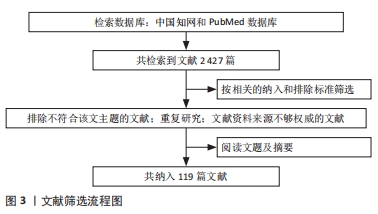
1.3 文献质量评估和数据的提取 共检索到中英文文献
2 427篇,选择与文章内容相关性大且新颖并具有价值的文章进行分析讨论,排除与研究目的相关性差及观点过时、重复的文献,按入选标准严格筛选后最终纳入119篇文献进行综述,其中英文文献114篇、中文文献5篇。文献筛选流程见图3。
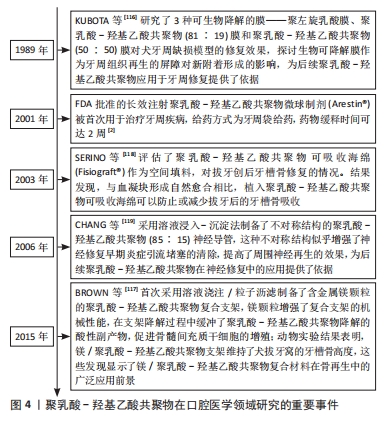
聚乳酸-羟基乙酸共聚物在口腔医学领域研究的重要事件,见图4[2,116-119]。
 从材料的角度来看,材料的性能直接取决于其组成、结构和加工技术。聚乳酸-羟基乙酸共聚物的多功能性是基于相对复杂的工艺技术,并在一定程度上限制了聚乳酸-羟基乙酸共聚物的临床转化。单一生物材料的应用往往存在一定的局限性,因此,在结构设计和引入物质进行修饰等方面需要研究人员进行慎重的考虑。此外,聚乳酸-羟基乙酸共聚物的临床应用还需要考虑生产的稳定性、价格的合理性和临床的可操作性。因此,探索更加便捷高效的生产模式将是聚乳酸-羟基乙酸共聚物的未来发展方向。
从材料的角度来看,材料的性能直接取决于其组成、结构和加工技术。聚乳酸-羟基乙酸共聚物的多功能性是基于相对复杂的工艺技术,并在一定程度上限制了聚乳酸-羟基乙酸共聚物的临床转化。单一生物材料的应用往往存在一定的局限性,因此,在结构设计和引入物质进行修饰等方面需要研究人员进行慎重的考虑。此外,聚乳酸-羟基乙酸共聚物的临床应用还需要考虑生产的稳定性、价格的合理性和临床的可操作性。因此,探索更加便捷高效的生产模式将是聚乳酸-羟基乙酸共聚物的未来发展方向。3.2 该综述的局限性 该综述概述了聚乳酸-羟基乙酸共聚物在口腔疾病治疗中应用的研究及进展,整理聚乳酸-羟基乙酸共聚物合成、理化性质和制备方法对口腔医学的影响,探讨了聚乳酸-羟基乙酸共聚物在口腔领域的应用及所面临的挑战。但由于文献筛选标准的差异,可能存在一些偏倚,遗漏了一些重要文献,导致对某些内容的描述不够详细。大量文献研究停滞于动物实验阶段,对临床应用参考价值有限,某些动物实验仍需进一步完善。文中的研究成果大多选择最新发表文献,但对于这些材料带来的长期修复效果未做足够的研究,因此,聚乳酸-羟基乙酸共聚物在口腔领域的临床转化是值得思考与解决的问题。
3.3 该综述的意义 随着技术的进步,聚乳酸-羟基乙酸共聚物在口腔医学领域的应用已经取得了显著进展。聚乳酸-羟基乙酸共聚物凭借其可控的降解性、良好的生物相容性、优良的可塑性等优点在口腔修复、种植、外科、内科等多个领域展现出巨大的应用潜力。聚乳酸-羟基乙酸共聚物具有可控的降解性,可以根据修复部位的不同而调整降解时间,使颌骨修复与支架降解达到平衡。聚乳酸-羟基乙酸共聚物可吸收屏障膜在治疗牙周炎和实现颌骨增量过程中避免了二次手术的影响。聚乳酸-羟基乙酸共聚物具有良好的生物相容性,这是组织修复中细胞增殖分化的前提。在口腔种植方面,聚乳酸-羟基乙酸共聚物涂层改善了钛植体表面的生物惰性,有利于形成“骨结合”。聚乳酸-羟基乙酸共聚物易于加工的性能可以制备成纳米粒子、微球、支架、屏障膜等,满足口腔不同部位的需要。3D打印技术的应用使得个性化制备成为可能,更有利于口腔组织的修复。聚乳酸-羟基乙酸共聚物能够负载多种疏水和亲水性活性物质,在龋病预防、根管消毒和盖髓治疗方面,相比活性因子的单独使用,聚乳酸-羟基乙酸共聚物粒子包裹的活性因子更有利于负载因子持久有效的释放,提高活性因子的临床疗效。然而,单纯的聚乳酸-羟基乙酸共聚物亲水性较差、细胞亲和力较低、机械性能不足。为了克服这些缺点,研究人员引入了各种改性策略,如将生物活性基团接枝到聚合物表面或将聚合物与其他材料混合,以赋予聚合物所需的性能,而将聚乳酸-羟基乙酸共聚物结合口腔干细胞(牙髓干细胞、牙周韧带干细胞和人脱落乳牙干细胞等)是未来组织工程发展的新方向。
该综述较为全面地回顾和总结了聚乳酸-羟基乙酸共聚物在口腔组织工程中的应用,从聚乳酸-羟基乙酸共聚物的优势性能出发,阐述聚乳酸-羟基乙酸共聚物在龋病防治、根管消毒、盖髓治疗、牙周治疗、种植体表面改性、颌骨修复和神经再生等方面的应用,并分析目前的挑战与未来前景,为其在口腔科学的深度研究提供新思路。 中国组织工程研究杂志出版内容重点:生物材料;骨生物材料;口腔生物材料;纳米材料;缓释材料;材料相容性;组织工程
 #br#
#br#
文题释义:
聚乳酸-羟基乙酸共聚物:由乳酸和羟基乙酸2种单体按不同比例随机聚合而成,这种人工合成的聚合物具有生物相容性、降解性、无毒、成囊和成膜等优点,被广泛应用于生物医用工程、制药及现代化工业领域。口腔医学:主要涉及口腔软硬组织,包括对组织生长发育及病变机制的研究,是口腔及颌面部疾病诊断、治疗、预防等方面的科学。
虽然聚乳酸-羟基乙酸共聚物已报道被广泛应用于组织工程和药物递送等方面,但罕见聚乳酸-羟基乙酸共聚物在口腔领域应用的综述。并且,聚乳酸-羟基乙酸共聚物在口腔疾病治疗的研究多局限于颌面骨缺损修复的应用,而在口腔其他部位的应用研究尚欠详细,对聚乳酸-羟基乙酸共聚物所具有的独特性能缺乏分析。因此,文章就聚乳酸-羟基乙酸共聚物的优秀性能与其在口腔疾病中的的应用展开研究,介绍聚乳酸-羟基乙酸共聚物的合成、理化性质、制备方法以及在口腔领域的应用,并简要讨论了聚乳酸-羟基乙酸共聚物的新趋势和未来方向,为进一步推动聚乳酸-羟基乙酸共聚物在口腔领域的发展提供参考。
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||