[1] RIEDEMANN HI, SCHMIDT MF, BARON JM. Therapy of pathological scars. J Dtsch Dermatol Ges. 2023;21(7):761-776.
[2] WANG ZC, ZHAO WY, CAO Y, et al. The Roles of Inflammation in Keloid and Hypertrophic Scars. Front Immunol. 2020;11:603187.
[3] LINGZHI Z, MEIRONG L, XIAOBING F. Biological approaches for hypertrophic scars. Int Wound J. 2020;17(2):405-418.
[4] LEE DE, AYOUB N, AGRAWAL DK. Mesenchymal stem cells and cutaneous wound healing: novel methods to increase cell delivery and therapeutic efficacy. Stem Cell Res Ther. 2016;7:37.
[5] ZHAO G, GE Y, ZHANG C, et al. Progress of Mesenchymal Stem Cell-Derived Exosomes in Tissue Repair. Curr Pharm Des. 2020;26(17):2022-2037.
[6] MASHOURI L, YOUSEFI H, AREF AR, et al. Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol Cancer. 2019;18(1):75.
[7] PEGTEL DM, GOULD SJ. Exosomes. Annu Rev Biochem. 2019;88:487-514.
[8] ZAREI F, ABBASZADEH A. Stem cell and skin rejuvenation. J Cosmet Laser Ther. 2018;20(3):193-197.
[9] SHIMBO K, MIYAKI S, ISHITOBI H, et al. Exosome-formed synthetic microRNA-143 is transferred to osteosarcoma cells and inhibits their migration. Biochem Biophys Res Commun. 2014;445(2):381-387.
[10] YU B, ZHANG X, LI X. Exosomes derived from mesenchymal stem cells. Int J Mol Sci. 2014;15(3):4142-4157.
[11] SHAO L, ZHANG Y, LAN B, et al. MiRNA-Sequence Indicates That Mesenchymal Stem Cells and Exosomes Have Similar Mechanism to Enhance Cardiac Repair. Biomed Res Int. 2017;2017:4150705.
[12] FARAZI TA, SPITZER JI, MOROZOV P, et al. miRNAs in human cancer. J Pathol. 2011; 223(2):102-115.
[13] MOBERGSLIEN A, SIOUD M. Exosome-derived miRNAs and cellular miRNAs activate innate immunity. J Innate Immun. 2014;6(1):105-110.
[14] MALET H, LORENTZEN E. Mechanisms of RNA recruitment by the exosome. RNA Biol. 2011;8(3):398-403.
[15] DENG H, SUN C, SUN Y, et al. Lipid, Protein, and MicroRNA Composition Within Mesenchymal Stem Cell-Derived Exosomes. Cell Reprogram. 2018;20(3):178-186.
[16] NANGOLE FW, AGAK GW. Keloid pathophysiology: fibroblast or inflammatory disorders? JPRAS Open. 2019;22:44-54.
[17] XU X, GU S, HUANG X, et al. The role of macrophages in the formation of hypertrophic scars and keloids. Burns Trauma. 2020;8:tkaa006.
[18] GORDON S, MARTINEZ FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32(5):593-604.
[19] XUE M, JACKSON CJ. Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring. Adv Wound Care (New Rochelle). 2015;4(3):119-136.
[20] NOVAK ML, KOH TJ. Macrophage phenotypes during tissue repair. J Leukoc Biol. 2013;93(6):875-881.
[21] ZHU Z, DING J, MA Z, et al. The natural behavior of mononuclear phagocytes in HTS formation. Wound Repair Regen. 2016;24(1):14-25.
[22] HSU YC, CHEN MJ, YU YM, et al. Suppression of TGF-β1/SMAD pathway and extracellular matrix production in primary keloid fibroblasts by curcuminoids: its potential therapeutic use in the chemoprevention of keloid. Arch Dermatol Res. 2010;302(10):717-724.
[23] CHEN J, ZENG B, YAO H, et al. The effect of TLR4/7 on the TGF-β-induced Smad signal transduction pathway in human keloid. Burns. 2013;39(3):465-472.
[24] SHEN D, HE Z. Mesenchymal stem cell-derived exosomes regulate the polarization and inflammatory response of macrophages via miR-21-5p to promote repair after myocardial reperfusion injury. Ann Transl Med. 2021;9(16):1323.
[25] TI D, HAO H, TONG C, et al. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J Transl Med. 2015;13:308.
[26] MORADI-CHALESHTORI M, BANDEHPOUR M, HEIDARI N, et al. Exosome-mediated miR-33 transfer induces M1 polarization in mouse macrophages and exerts antitumor effect in 4T1 breast cancer cell line. Int Immunopharmacol. 2021;90:107198.
[27] BERSCHNEIDER B, ELLWANGER DC, BAARSMA HA, et al. miR-92a regulates TGF-β1-induced WISP1 expression in pulmonary fibrosis. Int J Biochem Cell Biol. 2014;53:432-441.
[28] CHAE DK, BAN E, YOO YS, et al. MIR-27a regulates the TGF-β signaling pathway by targeting SMAD2 and SMAD4 in lung cancer. Mol Carcinog. 2017;56(8):1992-1998.
[29] DING F, YOU T, HOU XD, et al. MiR-21 regulates pulmonary hypertension in rats via TGF-β1/Smad2 signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23(9): 3984-3992.
[30] JAHANGIRIMOEZ M, MEDLEJ A, TAVALLAIE M, et al. Hsa-miR-587 Regulates TGFβ/SMAD Signaling and Promotes Cell Cycle Progression. Cell J. 2020;22(2):158-164.
[31] ZHAO S, NING Y, QIN N, et al. GAS5 regulates viability and apoptosis in TGF-β1-stimulated bronchial epithelial cells by regulating miR-217/HDAC4 axis. Genes Genomics. 2021;43(8):837-846.
[32] JIN J, JIA ZH, LUO XH, et al. Long non-coding RNA HOXA11-AS accelerates the progression of keloid formation via miR-124-3p/TGFβR1 axis. Cell Cycle. 2020; 19(2):218-232.
[33] 罗雅婷,解婧,许涛,等.人脐带间充质干细胞来源外泌体对小鼠压疮的治疗作用及机制[J].海军军医大学学报,2022,43(6):622-632.
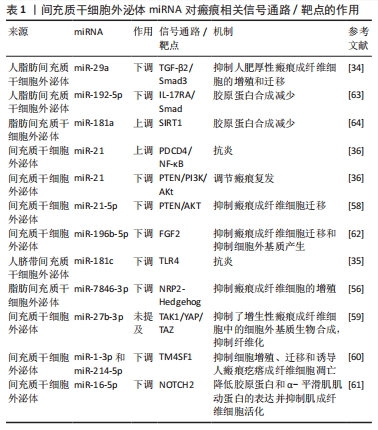
[34] YUAN R, DAI X, LI Y, et al. Exosomes from miR-29a-modified adipose-derived mesenchymal stem cells reduce excessive scar formation by inhibiting TGF-β2/Smad3 signaling. Mol Med Rep. 2021;24(5):758.
[35] LI X, LIU L, YANG J, et al. Exosome Derived From Human Umbilical Cord Mesenchymal Stem Cell Mediates MiR-181c Attenuating Burn-induced Excessive Inflammation. EBioMedicine. 2016;8:72-82.
[36] XIE J, WU W, ZHENG L, et al. Roles of MicroRNA-21 in Skin Wound Healing: A Comprehensive Review. Front Pharmacol. 2022;13:828627.
[37] AL-ATTAR A, MESS S, THOMASSEN JM, et al. Keloid pathogenesis and treatment. Plast Reconstr Surg. 2006;117(1):286-300.
[38] VERHAEGEN PD, VAN ZUIJLEN PP, PENNINGS NM, et al. Differences in collagen architecture between keloid, hypertrophic scar, normotrophic scar, and normal skin: An objective histopathological analysis. Wound Repair Regen. 2009;17(5): 649-656.
[39] SCHULTZ GS, DAVIDSON JM, KIRSNER RS, et al. Dynamic reciprocity in the wound microenvironment. Wound Repair Regen. 2011;19(2):134-148.
[40] LEE DE, TROWBRIDGE RM, AYOUB NT, et al. High-mobility Group Box Protein-1, Matrix Metalloproteinases, and Vitamin D in Keloids and Hypertrophic Scars. Plast Reconstr Surg Glob Open. 2015;3(6):e425.
[41] ZHAO X, KWAN JYY, YIP K, et al. Targeting metabolic dysregulation for fibrosis therapy. Nat Rev Drug Discov. 2020;19(1):57-75.
[42] MCKLEROY W, LEE TH, ATABAI K. Always cleave up your mess: targeting collagen degradation to treat tissue fibrosis. Am J Physiol Lung Cell Mol Physiol. 2013;304(11):L709-721.
[43] CALEY MP, MARTINS VL, O’TOOLE EA. Metalloproteinases and Wound Healing. Adv Wound Care (New Rochelle). 2015;4(4):225-234.
[44] 李文娟,陈伟,王海滨,等.不同发生时期的增生性瘢痕中基质金属蛋白酶及其抑制因子基因表达的变化[J].感染、炎症、修复,2006,7(1):14-17.
[45] FUJIWARA M, MURAGAKI Y, OOSHIMA A. Keloid-derived fibroblasts show increased secretion of factors involved in collagen turnover and depend on matrix metalloproteinase for migration. Br J Dermatol. 2005;153(2):295-300.
[46] GHAHARY A, GHAFFARI A. Role of keratinocyte-fibroblast cross-talk in development of hypertrophic scar. Wound Repair Regen. 2007;15 Suppl 1:S46-53.
[47] RUSSO B, BREMBILLA NC, CHIZZOLINI C. Interplay Between Keratinocytes and Fibroblasts: A Systematic Review Providing a New Angle for Understanding Skin Fibrotic Disorders. Front Immunol. 2020;11:648.
[48] NOWINSKI D, LYSHEDEN AS, GARDNER H, et al. Analysis of gene expression in fibroblasts in response to keratinocyte-derived factors in vitro: potential implications for the wound healing process. J Invest Dermatol. 2004;122(1):216-221.
[49] FUNAYAMA E, CHODON T, OYAMA A, et al. Keratinocytes promote proliferation and inhibit apoptosis of the underlying fibroblasts: an important role in the pathogenesis of keloid. J Invest Dermatol. 2003;121(6):1326-1331.
[50] MA T, FU B, YANG X, et al. Adipose mesenchymal stem cell-derived exosomes promote cell proliferation, migration, and inhibit cell apoptosis via Wnt/β-catenin signaling in cutaneous wound healing. J Cell Biochem. 2019;120(6): 10847-10854.
[51] YAN L, CAO R, LIU Y, et al. MiR-21-5p Links Epithelial-Mesenchymal Transition Phenotype with Stem-Like Cell Signatures via AKT Signaling in Keloid Keratinocytes. Sci Rep. 2016;6:28281.
[52] AZEVEDO ML, SILVEIRA RG, NEDEL F, et al. MicroRNAs expressed during normal wound healing and their associated pathways: A systematic review and bioinformatics analysis. PLoS One. 2023;18(4):e0281913.
[53] ZAKERI A, KHASEB S, AKHAVAN RAHNAMA M, et al. Exosomes derived from mesenchymal stem cells: A promising cell-free therapeutic tool for cutaneous wound healing. Biochimie. 2023;209:73-84.
[54] YANG C, LUO L, BAI X, et al. Highly-expressed micoRNA-21 in adipose derived stem cell exosomes can enhance the migration and proliferation of the HaCaT cells by increasing the MMP-9 expression through the PI3K/AKT pathway. Arch Biochem Biophys. 2020;681:108259.
[55] ZHU G, YANG X, PENG C, et al. Exosomal miR-532-5p from bone marrow mesenchymal stem cells reduce intervertebral disc degeneration by targeting RASSF5. Exp Cell Res. 2020;393(2):112109.
[56] WU D, LIU X, JIN Z. Adipose‐derived mesenchymal stem cells‐sourced exosomal microRNA‐7846‐3p suppresses proliferation and pro‐angiogenic role of keloid fibroblasts by suppressing neuropilin 2. J Cosmet Dermatol. 2023;22(8):2333-2342.
[57] ZHAO W, ZHANG H, LIU R, et al. Advances in Immunomodulatory Mechanisms of Mesenchymal Stem Cells-Derived Exosome on Immune Cells in Scar Formation. Int J Nanomedicine. 2023;18:3643-3662.
[58] YAN L, WANG LZ, XIAO R, et al. Inhibition of microRNA-21-5p reduces keloid fibroblast autophagy and migration by targeting PTEN after electron beam irradiation. Lab Invest. 2020;100(3):387-399.
[59] LI XM, YU WY, CHEN Q, et al. LncRNA TUG1 exhibits pro-fibrosis activity in hypertrophic scar through TAK1/YAP/TAZ pathway via miR-27b-3p. Mol Cell Biochem. 2021;476(8):3009-3020.
[60] XU M, SUN J, YU Y, et al. TM4SF1 involves in miR-1-3p/miR-214-5p-mediated inhibition of the migration and proliferation in keloid by regulating AKT/ERK signaling. Life Sci. 2020;254:117746.
[61] YAO Q, XING Y, WANG Z, et al. MiR-16-5p suppresses myofibroblast activation in systemic sclerosis by inhibiting NOTCH signaling. Aging (Albany NY). 2020; 13(2):2640-2654.
[62] YANG J, DENG P, QI Y, et al. NEAT1 Knockdown Inhibits Keloid Fibroblast Progression by miR-196b-5p/FGF2 Axis. J Surg Res. 2021;259:261-270.
[63] LI Y, ZHANG J, SHI J, et al. Exosomes derived from human adipose mesenchymal stem cells attenuate hypertrophic scar fibrosis by miR-192-5p/IL-17RA/Smad axis. Stem Cell Res Ther. 2021;12(1):221.
[64] CHEN J, YU W, XIAO C, et al. Exosome from adipose-derived mesenchymal stem cells attenuates scar formation through microRNA-181a/SIRT1 axis. Arch Biochem Biophys. 2023;746:109733.
[65] ZHANG M, CHEN H, QIAN H, et al. Characterization of the skin keloid microenvironment. Cell Commun Signal. 2023;21(1):207.
[66] MCGARRY T, BINIECKA M, VEALE DJ, et al. Hypoxia, oxidative stress and inflammation. Free Radic Biol Med. 2018;125:15-24.
[67] HONG L, JUNJIE C, PENGYU Z, et al. The mechanism of oxidative stress in keloid fibroblasts and the experimental study of early application of angiotensin-converting enzyme inhibitor. Indian J Dermatol Venereol Leprol. 2023:1-8. doi: 10.25259/IJDVL_323_2022. Epub ahead of print.
[68] WANG Q, YANG X, MA J, et al. PI3K/AKT pathway promotes keloid fibroblasts proliferation by enhancing glycolysis under hypoxia. Wound Repair Regen. 2023; 31(2):139-155.
[69] QIN H, ZHANG L, LI M, et al. EGR1/NOX4 pathway regulates oxidative stress and further facilitates fibrosis progression in keloids responses to TGF-β1. J Dermatol Sci. 2022;108(3):138-145.
[70] WU H, WANG J, MA H, et al. MicroRNA-21 inhibits mitochondria-mediated apoptosis in keloid. Oncotarget. 2017;8(54):92914-92925.
[71] ZHANG J, XU D, LI N, et al. Downregulation of microRNA-31 inhibits proliferation and induces apoptosis by targeting HIF1AN in human keloid. Oncotarget. 2017; 8(43):74623-74634.
[72] LV W, REN Y, WU M, et al. Identifying miRNA modules associated with progression of keloids through weighted gene co-expression network analysis and experimental validation in vitro. Burns. 2021;47(6):1359-1372.
[73] HE Y, ZHANG Z, YIN B, et al. Identifying miRNAs Associated with the Progression of Keloid through mRNA-miRNA Network Analysis and Validating the Targets of miR-29a-3p in Keloid Fibroblasts. Biomed Res Int. 2022;2022:6487989.
[74] POLTAVTSEVA RA, POLTAVTSEV AV, LUTSENKO GV, et al. Myths, reality and future of mesenchymal stem cell therapy. Cell Tissue Res. 2019;375(3):563-574. |