中国组织工程研究 ›› 2024, Vol. 28 ›› Issue (26): 4250-4256.doi: 10.12307/2024.436
• 组织构建综述 tissue construction review • 上一篇 下一篇
肠道菌群与骨质疏松及运动干预
杨启航1,蒲 锐1,2,陈子扬1,2,冷思逸1,宋永晶1,刘 辉3,杜光友1
- 长江大学,1教育与体育学院,2运动人体科学实验室,3医学部,湖北省荆州市 434023
-
收稿日期:2023-06-06接受日期:2023-08-07出版日期:2024-09-18发布日期:2023-10-07 -
通讯作者:杜光友,博士,副教授,硕士生导师,长江大学教育与体育学院,湖北省荆州市 434023 -
作者简介:杨启航,男,1999年生,湖北省襄阳市人,汉族,长江大学教育与体育学院在读硕士,主要从事运动健康促进、运动与慢性病干预研究。 -
基金资助:国家自然科学基金项目(82271514),项目负责人:刘辉;教育部人文社会科学研究基金项目(17YJA890004),项目负责人:杜光友;湖北省教育厅社科基金重点项目(16D024),项目负责人:杜光友
Intestinal flora and osteoporosis and exercise intervention
Yang Qihang1, Pu Rui1, 2, Chen Ziyang1, 2, Leng Siyi1, Song Yongjing1, Liu Hui3, Du Guangyou1
- 1College of Education and Sports Sciences, Yangtze University, Jingzhou 434023, Hubei Province, China; 2Human Science Laboratory of Exercise, Yangtze University, Jingzhou 434023, Hubei Province, China; 3Health Science Center, Yangtze University, Jingzhou 434023, Hubei Province, China
-
Received:2023-06-06Accepted:2023-08-07Online:2024-09-18Published:2023-10-07 -
Contact:Du Guangyou, PhD, Associate professor, Master’s supervisor, College of Education and Sports Sciences, Yangtze University, Jingzhou 434023, Hubei Province, China -
About author:Yang Qihang, Master candidate, College of Education and Sports Sciences, Yangtze University, Jingzhou 434023, Hubei Province, China -
Supported by:National Natural Science Foundation of China, No. 82271514 (to LH); the Ministry of Education Humanities and Social Sciences Research Foundation, No. 17YJA890004 (to DGY); Key Project of Social Science Fund of Education Department of Hubei Province, No. 16D024 (to DGY)
摘要:

文题释义:
肠道菌群:生活在人体肠道内所有微生物的总和,其种类超过 1 000种,遍布于十二指肠、小肠和结肠,总数量约为 1014个,它们与宿主和谐共生形成稳态。运动介导肠道菌群在骨质疏松中的调控作用:运动可通过调节肠道菌群及其代谢物分泌,从而发挥抑制骨细胞凋亡、抑制破骨细胞分化、促进成骨细胞分化及调节骨细胞营养代谢等作用,进而在防治骨质疏松中发挥有益效应。
背景:肠道菌群及其代谢物能参与骨质疏松的病理进程,在骨质疏松的诊断与治疗中发挥重要作用。此外,运动可调控肠道菌群进而影响骨质疏松的发生发展。
目的:总结肠道菌群对成骨细胞、破骨细胞和骨髓间充质干细胞等的作用与机制,探讨运动介导肠道菌群在调控骨质疏松中的潜在作用。方法:以“Intestinal flora,intestinal bacteria, metabolites of intestinal flora,bone metabolism, osteoporosis,exercise”和“肠道菌群,肠道细菌,肠道菌群代谢物,骨代谢,骨质疏松和运动”为关键词,检索PubMed和CNKI数据库1990-2023年间相关文献。
结果与结论:①肠道菌群丰度和多样性变化以及氧化三甲胺和胆汁酸等肠道菌群代谢物水平变化,能作为骨质疏松诊断的生物标记物。②肠道菌群失调可导致肠屏障功能障碍和产生过量脂多糖、氧化三甲胺,诱导分泌肿瘤坏死因子α等炎症细胞因子、激活核因子κB信号通路以及加剧氧化应激等,进而促进破骨细胞分化、诱导成骨细胞凋亡以及影响骨髓间充质干细胞的成骨迁移;重塑肠道菌群稳态能抑制炎症反应、下调氧化应激,进而抑制破骨细胞分化、促进成骨细胞分化以及调控骨髓间充质干细胞的成骨迁移,防治骨质疏松。③运动能调控肠道菌群稳态、改善肠屏障功能、促进短链脂肪酸和胆汁酸分泌、下调血清脂多糖水平、降低氧化应激,进而抑制骨细胞凋亡、抑制破骨细胞分化、促进成骨细胞分化和调节骨细胞营养代谢,从而发挥防治骨质疏松的潜力。
https://orcid.org/0000-0002-8590-3481(杨启航)
中国组织工程研究杂志出版内容重点:组织构建;骨细胞;软骨细胞;细胞培养;成纤维细胞;血管内皮细胞;骨质疏松;组织工程
中图分类号:
引用本文
杨启航, 蒲 锐, 陈子扬, 冷思逸, 宋永晶, 刘 辉, 杜光友. 肠道菌群与骨质疏松及运动干预[J]. 中国组织工程研究, 2024, 28(26): 4250-4256.
Yang Qihang, Pu Rui, Chen Ziyang, Leng Siyi, Song Yongjing, Liu Hui, Du Guangyou. Intestinal flora and osteoporosis and exercise intervention[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(26): 4250-4256.
2.2 肠道菌群与骨质疏松 研究表明肠道菌群与骨质疏松关系密切,肠道菌群在骨质疏松中可发挥关键诊疗作用,已成为骨质疏松诊疗的新靶点[11-12]。
2.2.1 肠道菌群在骨质疏松中的诊断作用 肠道菌群及其代谢物可作为骨质疏松诊断和预后的生物标记物。一项纳入全球多个国家和地区关于骨质疏松患者肠道菌群变化研究的系统评价发现,与健康人群相比, 骨质疏松患者肠道内乳酸杆菌、瘤胃球菌和拟杆菌等特定菌群分支相对丰度较高;此外,中国骨质疏松患者体内厚壁菌、另枝菌、巨单胞菌和厌氧菌等特定菌群丰度相对较低[13]。这一研究提示不同地区骨质疏松肠道菌群的组成不同,这可能与地区、生活方式和种族的不同有关。另有研究表明,骨骼状态改善与产短链脂肪酸菌群丰度增加呈显著正相关[14]。绝经妇女体内血清氧化三甲胺水平升高可提高其患骨质疏松和骨折风险的概率[15]。血清胆汁酸水平与骨矿物质密度呈正相关,与反映绝经妇女骨吸收的生物标志物βⅠ型胶原交联C-末端肽水平呈负相关[16]。上述研究表明,肠道菌群丰度和多样性变化以及氧化三甲胺和胆汁酸等肠道菌群代谢物水平变化能作为骨质疏松诊断的生物标记物。
LI等[12]建立雌激素缺乏小鼠模型,发现常规小鼠体内雌激素缺乏会诱导肠道菌群失调和肠道通透性增加,并上调破骨细胞因子分泌,促进破骨细胞分化,进而导致大量骨质流失;而采用抗生素处理之后,小鼠未出现由破骨细胞因子分泌诱导的骨吸收和小梁骨质流失。进一步的研究发现,该机制可能是肠道菌群参与机体炎症进而诱导骨质流失。研究发现与常规小鼠相比,无菌小鼠表现为骨量较低、生长发育缓慢等现象,而对无菌小鼠补充植物乳杆菌后骨骼呈现正常发育趋势[17]。SJ?GREN等[18]研究发现,与常规小鼠相比,无菌小鼠表现出与骨表面破骨细胞数量减少相关的骨量增加,而利用粪便移植技术将常规小鼠粪便中的肠道菌群定植入无菌小鼠体内,菌群移植上调了无菌小鼠骨髓中破骨细胞前体细胞的表达,使骨量趋于正常化。以上两项无菌对照实验表明,肠道菌群稳态能促进骨骼正常发育。但另有研究得出不一致的结论,QUACH等[19]利用管饲法将来源于人体和常规小鼠肠内的菌群定植入无菌小鼠体内,发现无菌小鼠表现出良好的菌群定植效果,但其定植前后有关骨代谢的参数几乎未发生改变。这3项研究结果的差异可能与实验受试的小鼠种类、菌群种类以及菌群移植方式不同有关。为深入了解其中的差异,需在未来研究中整合宏基因组学、转录组学以及蛋白质组学等不同方式探讨肠道菌群对骨骼健康影响的作用与机制。
综上所述,肠道菌群与骨质疏松密切相关,乳酸杆菌和瘤胃球菌特定菌群丰度变化以及肠道菌群代谢产物短链脂肪酸、胆汁酸和氧化三甲胺的水平变化与破骨细胞数量及分化、破骨细胞前体细胞表达、骨密度和骨转换生物标志物水平变化等骨质疏松病理发展标志相关,可作为其诊断的生物标记物,反映出骨质疏松严重程度。据此,肠道菌群及其代谢物的检测能作为间接监控宿主健康状态、预测和评价宿主骨质疏松的生物标记物之一。但目前相关研究由于实验设计的差异导致结果不一致,后续研究仍需对特定肠道菌群及其代谢物的诊断作用进行验证,探求骨质疏松诊断的普遍性和精确性选择。
2.2.2 肠道菌群在骨质疏松中的治疗作用 机体骨代谢平衡是由成骨细胞介导的骨形成与破骨细胞介导的骨吸收来维持的,并受雌激素、甲状旁腺激素、维生素D以及炎性细胞因子等调节,当破骨细胞对骨的吸收逐渐增加、成骨细胞的成骨作用逐渐减弱时,将会导致骨量迅速流失和骨质疏松的发生[1]。肠道菌群及其代谢物能调节破骨细胞、成骨细胞和骨髓间充质干细胞的分化增殖与凋亡,进而影响骨质疏松病理进程。
(1)肠道菌群与破骨细胞:肠道菌群失调会导致肠道屏障功能障碍和分泌过量的脂多糖和氧化三甲胺,进而加剧炎症反应和氧化应激,诱导破骨细胞分化形成。研究发现,肠道菌群失调会导致肠内革兰阴性细菌分泌过量的脂多糖,诱发肠道炎症,导致肠上皮紧密连接出现病理变化,使得肠屏障通透性变大形成“肠漏”,从而使得脂多糖从肠内进入到体循环,并借助血液流动与骨免疫细胞相互作用上调核因子κB信号通路表达,同时大量释放肿瘤细胞因子α、白细胞介素1β和破骨细胞分化因子等炎症细胞因子,促进破骨细胞分化,诱导骨关节炎和骨质流失的发生[20]。另有研究表明,破骨细胞分化因子/骨保护素和核因子κB等信号通路的表达能促进破骨细胞形成[21-22]。此外,肠道菌群失调产生过量的氧化三甲胺能诱导氧化应激和加剧炎症反应,进而上调降钙素受体、肿瘤坏死因子受体相关蛋白6、树突表达特异性7跨膜蛋白、酸性磷酸酶5、c-fos原癌基因蛋白和NFATc1等破骨细胞相关蛋白表达,加速骨质流失[23]。这一研究提示,高水平的氧化三甲胺会导致炎症反应和氧化应激,促进破骨细胞分化。另有研究证明,低水平的氧化三甲胺能下调促炎细胞因子水平,从而参与到骨质疏松的治疗中。CHEN等[24]研究发现,补充金纳米球可重塑肠道菌群稳态和降低氧化三甲胺相关代谢水平,并抑制促炎细胞因子分泌,进而减轻由雌激素缺乏引起的骨质流失。这一研究表明,维持肠道菌群-氧化三甲胺的动态平衡可在治疗骨质疏松中发挥关键作用。以上研究提示,肠道菌群失调会产生高水平脂多糖和氧化三甲胺,从而上调肿瘤细胞因子α等炎症细胞因子分泌水平和加剧氧化应激,进而上调破骨细胞分化因子/骨保护素和核因子κB等信号通路以及降钙素受体、肿瘤坏死因子受体相关蛋白6等破骨细胞相关蛋白表达,促进破骨细胞形成。
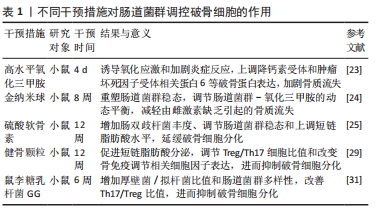
调节肠道菌群稳态和补充短链脂肪酸能通过抑制炎症反应和介导免疫抑制破骨细胞分化。LIU等[25]发现,补充硫酸软骨素可显著调节肠道菌群稳态和上调短链脂肪酸水平,进而减轻炎症反应,抑制破骨细胞形成。一方面,调节肠道菌群稳态能改善肠屏障功能和避免脂多糖“肠漏”的发生,减轻炎症反应;另一方面,增加的短链脂肪酸能激活G 蛋白偶联受体41/43/109A受体或通过抑制组蛋白脱乙酰酶活性来调节机体炎症信号通路的表达,抑制炎症反应[26]。另有研究表明,免疫细胞CD4细胞亚群中Treg和Th17对于维持骨稳态至关重要,Treg/Th17细胞及其相关细胞因子白细胞介素17和转化生长因子β能调节骨保护素/破骨细胞分化因子受体信号轴,进而调控破骨细胞分化[27-28]。SUN等[29]研究发现,服用健骨颗粒可调节卵巢摘除绝经大鼠肠道菌群稳态、促进短链脂肪酸分泌、调节Treg/Th17细胞比值和改变骨免疫调节相关细胞因子表达,进而抑制破骨细胞分化,有效防止骨质流失。补充鼠李糖乳杆菌GG能提高厚壁菌/拟杆菌比值、促进短链脂肪酸分泌、上调紧密连接蛋白表达和改善Th17/Treg比值,从而抑制破骨细胞分化[30]。此外,短链脂肪酸可直接作用于破骨细胞,诱导破骨细胞代谢重编并降低破骨细胞内氧化磷酸化水平,进而下调破骨细胞基因表达,抑制破骨细胞分化[31]。以上研究提示,调节肠道菌群稳态和补充短链脂肪酸能通过修复肠屏障损伤以及下调炎症信号通路表达来抑制炎症反应和介导骨免疫调节,改善Treg/Th17细胞比值并调控破骨细胞分化因子表达,进而抑制破骨细胞分化。此外,短链脂肪酸还可直接下调破骨细胞基因表达,抑制破骨细胞分化。这些研究为以肠道菌群及其代谢物调节破骨细胞进而治疗骨质疏松提供了新的机制或药物靶点,不同干预措施对肠道菌群调控破骨细胞的作用,见表1。
(2)肠道菌群与成骨细胞:肠道菌群失调会激活核因子κB信号通路以及诱导下丘脑-垂体-肾上腺轴分泌紊乱,进而加速成骨细胞凋亡。研究发现,肠道菌群失调与肠屏障功能障碍会导致大量脂多糖从肠道渗漏到体循环中并加剧氧化应激,进而激活核因子κB信号通路诱导成骨细胞凋亡,从而抑制骨形成[32-34]。此外,肠道菌群能影响下丘脑-垂体-肾上腺轴应激反应的发生,而当肠道菌群失调时,在下丘脑-垂体-肾上腺轴应激反应作用下会导致皮质醇释放异常,使得皮质醇和外源性糖皮质激素释放过度,加速成骨细胞凋亡[35-37]。以上研究提示,肠道菌群失调会引起炎症信号通路表达和皮质醇释放异常,加速成骨细胞凋亡。
重塑肠道菌群稳态能调节骨形态发生蛋白-转化生长因子β信号通路表达和调控胰岛素样生长因子1与血清素等激素分泌,进而促进成骨细胞分化。HONG等[38]研究发现,补充肉桂酸可增加盲肠内菌群多样性和上调乳酸杆菌和脱硫弧菌科等特定菌群丰度,并激活骨形态发生蛋白-转化生长因子β信号通路,从而上调成骨特异性转录因子表达,促进成骨细胞分化。此外,XIAO等[39]将健康小鼠的肠道菌群定植入骨质流失小鼠体内,发现定植能改善小鼠的肠道菌群失调,并上调血清和骨骼中的胰岛素样生长因子1水平,改善小鼠成骨细胞功能。YADAV等[40]研究发现,肠道菌群特异性色氨酸羟化酶1基因表达失活可降低血清素水平,进而加速骨形成并防止卵巢切除术诱导的骨质流失。另有研究表明,补充威廉链球菌来源的木脂素可通过抑制肠道特异性色氨酸羟化酶1蛋白和调节肠道微生物群组成来抑制血清素合成,从而间接发挥骨骼保护作用[41]。以上两项研究提示,肠道菌群介导胰岛素样生长因子1和血清素合成进而调控成骨细胞功能,然而目前其调控机制尚不清楚,仍需进一步研究。由上可知,重塑肠道菌群稳态可上调成骨特异性转录因子表达以及介导内分泌,调节胰岛素样生长因子1和血清素等激素分泌水平,进而促进成骨细胞分化。
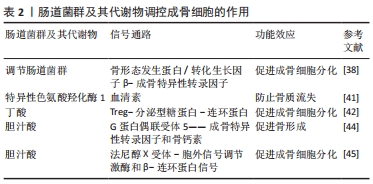
肠道菌群代谢物丁酸和胆汁酸可作为促进成骨细胞分化的积极调控因子。LI等[42]研究发现,对幼鼠补充丁酸能刺激骨形成发生,进而增加骨小梁体积,进一步的研究发现,丁酸能上调Treg数量、激活分泌型糖蛋白/β-连环蛋白信号通路表达,进而促进成骨细胞分化。LI等[43]研究发现,胆汁酸通过激活G蛋白偶联受体5和法尼醇X受体表达,减少小鼠雌激素依赖性骨质流失。另有研究表明,G蛋白偶联受体5在成骨细胞样细胞系MC3T3-E1中可刺激成骨特异性转录因子表达,并上调碱性磷酸酶和骨钙素等与基质矿化和成骨细胞分化有关的基因表达,进而促进骨形成[44]。而法尼醇X受体能上调成骨特异性转录因子表达,并上调胞外信号调节激酶活性和增强β-连环蛋白信号传导,进而促进成骨细胞分化[45]。上述研究提示,肠道菌群代谢物丁酸和胆汁酸能激活分泌型糖蛋白/β-连环蛋白信号通路和上调成骨细胞分化有关的基因表达等不同方式促进成骨细胞分化,防治骨质流失。肠道菌群及其代谢物调控成骨细胞的作用,见表2。
(3)肠道菌群与骨髓间充质干细胞:骨髓间充质干细胞作为一种多能干细胞可分化为成骨细胞、软骨细胞和脂肪细胞等[46]。研究表明,调节骨髓间充质干细胞的迁移方向能重建骨组织微观结构,从而在骨重塑和骨再生过程中促进骨形成[47]。一项临床研究发现,慢性缺氧会降低肠道微菌多样性和减少肠道内乳酸杆菌丰度、加剧氧化应激,进而诱导骨髓间充质干细胞过早衰老,而在缺氧大鼠模型中补充乳酸杆菌能有效改善由缺氧引起的骨髓间充质干细胞过早衰老[48]。这一研究提示,肠道菌群能影响骨髓间充质干细胞功能,其稳态失调会上调氧化应激进而诱导骨髓间充质干细胞过早衰老,补充益生菌乳酸杆菌能改善由缺氧引起的骨髓间充质干细胞过早衰老。另有研究发现,肠道菌群代谢物氧化三甲胺能激活核因子κB信号通路和提高氧化应激水平,抑制骨髓间充质干细胞向成骨细胞分化,并促使骨髓间充质干细胞向脂肪细胞分化[49]。由上可知,肠道菌群紊乱及其代谢物氧化三甲胺的过量分泌能加剧氧化应激,诱导骨髓间充质干细胞早衰、抑制其增殖以及诱导其向脂肪细胞分化,不利于骨骼健康。
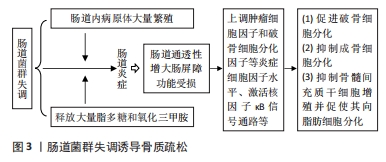
补充益生菌动物乳杆菌-EV可作为调节骨髓间充质干细胞治疗骨质疏松的潜在治疗方法。研究发现,动物乳杆菌-EV分泌的功能蛋白可促进与血管生成、成骨细胞分化和抗细胞凋亡相关的蛋白进入到内皮细胞和骨细胞中,促进血管内皮生成,进而增强骨髓间充质干细胞成骨分化和抑制骨细胞凋亡[50]。此外,LI等[51]研究发现,桃红四物汤能通过调节肠道菌群结构与功能、上调血管内皮生长因子分泌、激活黏附激酶信号通路表达,进而促进骨髓间充质干细胞的发育和分化。由上可知,补充动物乳杆菌-EV和桃红四物汤能介导肠道菌群来促进骨髓间充质干细胞的发育、抑制骨细胞凋亡和促进骨髓间充质干细向成骨细胞转化,从而增强骨质。肠道菌群失调诱导骨质疏松,见图3。
2.3 运动调控肠道菌群研究进展
2.3.1 有氧运动对肠道菌群的影响 多数动物实验证实,有氧运动对宿主肠道菌群有益。QUEIPO-ORTU?O等[52]研究表明,短期(6 d)自主跑轮运动可显著增加小鼠乳酸杆菌、双歧杆菌和球状芽孢杆菌-直肠芽孢杆菌等益生菌丰度,并使肠内丁盐酸浓度上升。有研究表明,6周跑步机运动可降低小鼠体内杆菌属丰度、提高厚壁菌属丰度[53];4周中等强度的跑步机运动可重塑小鼠肠道菌群,增加其多样性并促进丁酸单胞菌、普雷沃氏菌和阿克曼氏菌等与机体代谢有益菌群丰度增加[54]。此外,长期适度跑步机运动可显著降低肥胖程度,增加结肠肠道菌群多样性和丰度[55]。另有人体试验表明,个体心肺水平与其肠道菌群多样性呈正相关,心肺水平较高者其肠道内梭状芽胞杆菌、玫瑰杆菌、乳杆菌科和丹毒科属等产丁酸盐菌菌群丰度较高,提示运动可通过重塑肠道菌群进而促进肠道健康[56]。6周有氧运动可增加肠道菌群多样性和肠道菌群代谢物短链脂肪酸水平,但中止运动后由运动引起的肠道菌群变化在很大程度上被逆转[57]。与久坐不动的人群相比,橄榄球运动员的肠道菌群α多样性和与健康相关属阿克曼氏菌的相对丰度更高[58]。MOTIANI等[59]对26名久坐不动的中年胰岛素抵抗受试者进行2周自行车运动干预,发现短期运动训练可改善门和属水平的肠道菌群,降低厚壁菌/拟杆菌属比值,并降低体内内毒素血症标志物水平,改善胰岛素敏感性和宿主新陈代谢。此外,有日本学者对128名绝经妇女进行6个月的大豆异黄酮与步行联合干预,发现异黄酮可预防骨质流失,对骨骼的有益作用可能取决于受试者肠道菌群产生雌马酚的能力[60]。
综上所述,跑步机运动、橄榄球运动、自行车以及步行等有氧运动均可重塑肠道菌群,增加肠道菌群多样性和丰度,增加乳酸杆菌、双歧杆菌和阿克曼氏菌等与机体代谢有益菌群丰度,但运动停止后由运动引起的肠道菌群变化在很大程度上可能被逆转。目前尚缺乏有氧运动改善肠道菌群及其代谢物的具体机制研究。
2.3.2 无氧运动对肠道菌群的影响 CASTRO等[61]对Wistar大鼠进行12周的抗阻运动干预,发现抗阻运动具有增加肠道菌群多样性并改善其生物学功能的潜力。另有研究表明,12周的高强间歇运动可增加肠道菌群多样性和丰度,下调Toll样受体4表达,从而预防高脂膳食对肠组织的损伤[62]。此外,DENOU等[63]对肥胖雄性小鼠进行6周高强间歇运动干预后,发现肠道菌群α多样性和拟杆菌/厚壁菌比值提高。人体试验表明,10周的抗阻训练可提高蛋白摄入量并重塑肠道菌群[64]。DUPUIT等[65]对17名肥胖女性受试者进行12周的抗阻与高强间歇运动联合训练,与对照组相比,运动组可显著改善肠道菌群组成。但RETTEDAL等[66]研究发现,3周高强间歇运动产生的胰岛素敏感性改善与粪球菌属、经黏液真杆菌属和毛螺菌属等菌群丰度变化有关,但未对瘦和超重男性的肠道菌群结构产生很大影响。
综上所述,无氧运动对肠道菌群影响存在异质性,在动物实验和人体试验中抗阻运动都能重塑肠道菌群,增加肠道菌群多样性及丰度、有益菌群丰度等。而高强间歇运动对肠道菌群的影响却存在差异,一些研究发现,高强间歇运动能增加肠道菌群多样性和丰度,预防肠道损伤;而另一些研究发现,高强间歇运动对机体造成的有益影响与某些特定菌群变化有关,但未对肠道菌群总体的组成与结构产生影响。这种研究结果的差异可能与研究对象、方法、运动强度、运动负荷、运动方式的不同有关,需要进一步深入研究。
2.4 运动介导肠道菌群改善骨代谢的潜在作用 定期运动已被证明有益于骨骼健康。相关研究表明,运动可通过调控肠道菌群抑制骨细胞凋亡、抑制破骨分化、促进成骨细胞分化以及调节骨细胞营养代谢等潜在途径改善骨骼健康。以肠道菌群为靶点进行运动干预可作为改善骨质疏松的潜在方向。
2.4.1 运动调控肠道菌群抑制骨细胞凋亡 骨细胞凋亡是影响骨代谢失衡、造成骨质疏松的危险因素,抑制成骨细胞凋亡对于预防骨质流失具有重要意义。LEE等[67]研究发现,补充益生菌发酵产生的乳制品能调节肠道菌群、下调血清脂多糖水平,从而使骨质疏松大鼠核因子κB信号通路和涉及骨细胞凋亡的基因表达趋于正常化,进而改善骨质疏松。这一研究提示,益生菌调控肠道菌群稳态可下调血清脂多糖和氧化应激水平、抑制核因子κB信号通路,进而抑制成骨细胞凋亡。而运动能介导肠道菌群下调脂多糖-Toll样受体4/核因子κB炎症信号通路表达和降低机体氧化应激,进而抑制骨细胞凋亡。
研究表明,8周的转轮运动可重塑小鼠肠道菌群稳态和改善肠屏障功能进而提高循环系统中脂多糖清除率,抑制炎症因子表达,并增加软骨厚度和改善软骨变性,减轻骨关节炎的严重程度[68]。与之类似,刘洋[69]研究发现,高脂饮食可通过调控小鼠肠道菌群从而上调血浆脂多糖水平,进而过度激活膝关节软骨脂多糖-Toll样受体4/核因子κB炎症信号通路表达,参与到肥胖性骨关节炎的病理进程中,而4周的转轮运动可通过改善肠道菌群结构并抑制脂多糖-Toll样受体4/核因子κB炎症信号通路活化,进而减轻小鼠肥胖性骨关节炎。由上可知,有氧运动可通过调节肠道菌群多样性与丰度和改善肠屏障功能,提高循环系统中脂多糖清除率并抑制核因子κB信号通路激活,进而抑制骨细胞凋亡。此外,运动介导肠道菌群能降低机体氧化应激水平。梁家琪等[70]发现高强度自行车滚轮运动能增加乳杆菌和双歧杆菌等益生菌丰度,改善肠道菌群稳态,并调节超氧化物歧化酶、过氧化氢酶及非酶系统谷胱甘肽等抗氧化酶的活性,从而消除氧化应激,预防机体病原体入侵和改善肌肉组织损伤。这一研究提示运动能介导肠道菌群减轻氧化应激,为运动调控肠道菌群减轻氧化应激进而抑制骨细胞凋亡提供了相关理论支撑。
动物实验表明,转轮运动能重塑肠道菌群稳态、下调炎症信号通路表达,进而抑制骨细胞凋亡;高强度自行车滚轮运动能调控肠道菌群减轻氧化应激抑制骨细胞凋亡。但目前缺乏临床试验来验证这一机制,相关研究多集中在动物实验领域,因此仍需要运动调控肠道菌群抑制骨细胞凋亡进行深入的临床研究,进而为治疗骨质疏松提供更多坚实的理论及实验依据。
2.4.2 运动调控肠道菌群抑制破骨细胞分化 抑制破骨细胞增殖分化是降低骨吸收预防骨质疏松的有效措施之一。厚壁菌/拟杆菌比值是影响破骨细胞分化关键因素[11]。MCCABE等[71]建立肥胖小鼠模型,发现14周的自主转轮运动能降低小鼠厚壁菌/拟杆菌比值和增加肠双歧杆菌科丰度,同时增加骨量、改善骨骼力学性能和减少骨质流失,进而重塑肠道菌群稳态和预防骨质疏松。这一研究提示,步行可通过调节微生物群组成、降低厚壁菌/拟杆菌比值进而预防高脂饮食诱导的骨质流失。
此外,运动还可调节肠道菌群稳态和上调短链脂肪酸水平,调控免疫系统,进而抑制破骨细胞分化。维持Treg和Th17细胞比值平衡是保持骨稳态的重要因素,在体外Treg可直接抑制破骨细胞分化,而Th17细胞通过诱发局部炎症刺激破骨细胞分化因子表达,进而促进破骨细胞形成[27,72]。CHEN等[73]研究发现,力量运动通过改善肠道菌群稳态、改善肠道屏障功能并介导神经免疫调节,抑制Th17细胞分化和增加Treg细胞数量,进而降低脑脊髓炎严重程度和增加淋巴细胞。此外,短链脂肪酸可调节Treg细胞分化和分泌白细胞介素4、白细胞介素10和转化生长因子β1,进而抑制破骨形成[74]。HUANG等[75]研究发现,耐力运动可改善肠道菌群稳态和促进短链脂肪酸分泌增加,进而抑制炎症和免疫细胞趋化反应。另有研究发现,将职业橄榄球运动员和久坐不动受试者的肠道菌群进行对比,发现橄榄球运动能调节肠道菌群代谢物短链脂肪酸水平[58]。以上研究提示,运动能上调短链脂肪酸分泌水平、调节Th17∶Treg细胞比值,进而抑制破骨细胞分化,防治骨质流失。
由此可见,适度运动对于肠道菌群在抑制破骨细胞分化方面具有重要意义,不同运动方式介导肠道菌群抑制破骨细胞分化的机制不同。自主转轮运动通过调节厚壁菌/拟杆菌比值进而抑制破骨细分化;抗阻运动能改善肠道菌群、抑制 Th17细胞分化和增加Treg细胞数量,从而抑制破骨细胞形成;而耐力运动则通过上调短链脂肪酸水平、调节Treg细胞分化、抑制破骨形成。但目前缺乏对不同运动负荷调控肠道菌群抑制破骨细胞分化来达到缓解骨质疏松的系统性研究,因此需要进一步探讨不同类型运动介导肠道菌群对成骨细胞的影响,这些问题的揭示将有助于更好地理解运动调节肠道菌群预防骨质流失的途径。
2.4.3 运动调控肠道菌群促进成骨细胞分化 运动能介导肠道菌群稳态调节内分泌激素水平,进而刺激成骨细胞活性和促进成骨细胞增殖。有日本学者对中老年健康绝经后妇女进行为期6个月的异黄酮联合步行干预,发现异黄酮与步行对改变绝经后日本女性的脂质代谢和身体成分具有协同作用,能有效刺激成骨细胞活性、改善骨质流失[60,76]。此外,瘦素能刺激成骨细胞的增殖[77]。研究表明,有氧运动能增加胰岛素敏感性、调节瘦素水平和降低局部和全身和血清内毒素水平,改善肠道菌群稳态失调和骨关节炎[78]。QUEIPO-ORTU?O等[52]研究发现,不同膳食营养联合运动可增加肠道菌群多样性,提高双歧杆菌、乳酸杆菌、普雷沃氏菌和梭状芽胞杆菌等特定菌群丰度,并上调外周血清瘦素水平和下调生长素释放肽水平,进而改善肠道菌群稳态和调控宿主代谢和饱腹感。以上研究提示,运动介导肠道菌群调节雌激素、瘦素和生长素释放肽等激素分泌水平,促进成骨细胞分化。
此外,胆汁酸和丁酸是促进成骨细胞分化的重要调节因子。MEISSNER等[79]研究发现,12周的自主转轮运动可上调胆汁酸分泌水平。此外,运动还可促进丁酸分泌促进成骨细胞分化。与久坐不动的小鼠相比,8周跑步机运动可促进小鼠体内丙酸和丁酸代谢富集[80]。ALLEN等[57]对久坐不动的不同体型成年人进行为期6周的有氧运动干预,发现有氧运动可上调体内产丁酸菌菌群丰度和丁酸水平。6周的耐力运动可增加久坐和超重女性肠道内疣状微生物、疣状微生物科、阿克曼菌和毛螺菌的相对丰度,促进丁酸分泌[81]。以上研究提示,运动可促进肠道菌群代谢物胆汁酸和丁酸富集,进而促进成骨细胞分化。
综上所述, 步行能调控肠道菌群介导内分泌能对成骨细胞分化产生积极作用,而关于运动介导肠道菌群调节内分泌以及调控肠道菌群代谢物水平进而促进成骨细胞分化的相关研究较少,缺乏不同运动方式介导肠道菌群促进成骨细胞分化的横向比较,在这一新领域仍需进一步深入探讨。
2.4.4 运动调控肠道菌群调节骨细胞营养代谢 钙和维生素D是骨骼健康的关键营养素,缺乏钙和维生素D会导致骨骼发育不良[82]。而运动可调控肠道菌群改善肠屏障功能,促进骨细胞营养吸收。研究表明,肠道菌群能调节黏膜厚度和渗透性,改善肠屏障功能,从而有利于蛋白质、矿物质和维生素的吸收利用,进而改善骨细胞营养代谢[83]。一项有关人体的随机对照试验发现,补充合生元联合力量运动干预可增加肠道菌群多样性、促进短链脂肪酸分泌增加和调节免疫反应,降低肠道pH值,进而促进肠道对钙的吸收,改善身体成分和骨骼健康[84]。长期适度跑步机运动可显著降低小鼠肥胖程度,调节结肠肠道菌群多样性和丰度,有效激活AMP依赖的蛋白激酶/尾侧型同源转录因子2信号通路表达,改善高脂饮食诱导的肥胖小鼠肠道屏障损伤[55]。适度游泳运动可改善肠道菌群稳态、减少细菌异位和上调抗菌肽表达,进而减轻慢性应激诱导的肠道屏障功能障碍[85]。有氧运动能改善肠道菌群的组成与丰度,并上调紧密连接蛋白表达和抑制炎症因子分泌,进而减轻由氟化物诱导的小鼠肠道损伤和肠道菌群紊乱[86]。此外,短链脂肪酸能促进骨骼对钙的吸收[87]。而运动可促进短链脂肪酸的分泌[58,75]。
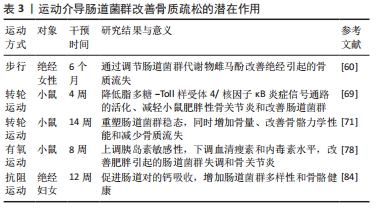
以上研究提示,抗阻运动可改善肠屏障功能进而促进骨细胞对蛋白质、矿物质和维生素的吸收利用,促进骨骼健康。持续的有氧运动已被证明可增加微生物群多样性、保护胃肠道屏障功能、抑制炎症反应。但运动过量会导致肠道通透性增大,增加循环中脂多糖含量。不同类型的运动训练方案或不同运动负荷对肠道菌群的影响存在差异,过量负荷会损伤肠胃道,从而不利于骨细胞营养吸收。但目前尚未见报道对不同运动方式或负荷调控肠道菌群发挥促进骨细胞营养吸收的具体机制进行系统性的描述,因此需要进一步探讨不同类型运动方案和运动负荷对肠道菌群的影响,这些问题的揭示将有助于更好地理解运动调节肠道菌群发挥营养吸收的途径和具体机制。运动介导肠道菌群改善骨质疏松的潜在作用,见表3。
| [1] KENDLER DL, MARIN F, ZERBINI CAF, et al. Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet. 2018;391(10117):230-240. [2] WALDBAUM JDH, XHUMARI J, AKINSUYI OS, et al. Association between Dysbiosis in the Gut Microbiota of Primary Osteoporosis Patients and Bone Loss. Aging Dis. 2023. doi: 10.14336/AD.2023.0425 [3] 杨启航,蒲锐,陈子扬,等.肠道菌群代谢物在肥胖调控中的作用与机制[J].中国组织工程研究,2024,28(2):308-314. [4] AKINSUYI OS, ROESCH LFW. Meta-Analysis Reveals Compositional and Functional Microbial Changes Associated with Osteoporosis. Microbiol Spectr. 2023;11(3): e0032223. [5] INCHINGOLO AM, GARGIULO ISACCO C, INCHINGOLO AD, et al. The human microbiota key role in the bone metabolism activity. Eur Rev Med Pharmacol Sci. 2023;27(6):2659-2670. [6] 佟喆,张振南,于潼.运动疗法防治骨质疏松症机制的研究进展[J].中国骨质疏松杂志,2022,28(10):1556-1560. [7] SENDER R, FUCHS S, MILO R. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell. 2016;164(3):337-340. [8] GOMAA EZ. Human gut microbiota/microbiome in health and diseases: a review. Antonie Van Leeuwenhoek. 2020;113(12):2019-2040. [9] VáZQUEZ-BAEZA Y, CALLEWAERT C, DEBELIUS J, et al. Impacts of the Human Gut Microbiome on Therapeutics. Annu Rev Pharmacol Toxicol. 2018;58:253-270. [10] CONTINO KF, YADAV H, SHIOZAWA Y. The gut microbiota can be a potential regulator and treatment target of bone metastasis. Biochem Pharmacol. 2022; 197:114916. [11] YUAN Y, YANG J, ZHUGE A, et al. Gut microbiota modulates osteoclast glutathione synthesis and mitochondrial biogenesis in mice subjected to ovariectomy. Cell Prolif. 2022;55(3):e13194. [12] LI JY, CHASSAING B, TYAGI AM, et al. Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J Clin Invest. 2016;126(6): 2049-2063. [13] HUANG R, LIU P, BAI Y, et al. Changes in the gut microbiota of osteoporosis patients based on 16S rRNA gene sequencing: a systematic review and meta-analysis. J Zhejiang Univ Sci B. 2022;23(12):1002-1013. [14] CZERNIK PJ, GOLONKA RM, CHAKRABORTY S, et al. Reconstitution of the host holobiont in germ-free born male rats acutely increases bone growth and affects marrow cellular content. Physiol Genomics. 2021;53(12):518-533. [15] LIU Y, GUO YL, MENG S, et al. Gut microbiota-dependent Trimethylamine N-Oxide are related with hip fracture in postmenopausal women: a matched case-control study. Aging (Albany NY). 2020;12(11):10633-10641. [16] ZHAO YX, SONG YW, ZHANG L, et al. Association between bile acid metabolism and bone mineral density in postmenopausal women. Clinics (Sao Paulo). 2020; 75:e1486. [17] SCHWARZER M, MAKKI K, STORELLI G, et al. Lactobacillus plantarum strain maintains growth of infant mice during chronic undernutrition. Science. 2016; 351(6275):854-857. [18] SJÖGREN K, ENGDAHL C, HENNING P, et al. The gut microbiota regulates bone mass in mice. J Bone Miner Res. 2012;27(6):1357-1367. [19] QUACH D, COLLINS F, PARAMESWARAN N, et al. Microbiota Reconstitution Does Not Cause Bone Loss in Germ-Free Mice. mSphere. 2018;3(1):e00545-17. [20] LIU S, LI G, XU H, et al. “Cross-talk” between gut microbiome dysbiosis and osteoarthritis progression: a systematic review. Front Immunol. 2023;14:1150572. [21] LI C, HUANG Q, YANG R, et al. Gut microbiota composition and bone mineral loss-epidemiologic evidence from individuals in Wuhan, China. Osteoporos Int. 2019;30(5):1003-1013. [22] 袁剑,孔令俊,丁海霞,等.肿瘤坏死因子-α调控骨质疏松症作用机制研究[J].中国骨质疏松杂志,2023,29(3):426-430+436. [23] WANG N, HAO Y, FU L. Trimethylamine-N-Oxide Promotes Osteoclast Differentiation and Bone Loss via Activating ROS-Dependent NF-κB Signaling Pathway. Nutrients. 2022;14(19):3955. [24] CHEN Y, YANG C, DAI Q, et al. Gold-nanosphere mitigates osteoporosis through regulating TMAO metabolism in a gut microbiota-dependent manner. J Nanobiotechnol. 2023;21(1):125. [25] LIU T, YU H, WANG S, et al. Chondroitin sulfate alleviates osteoporosis caused by calcium deficiency by regulating lipid metabolism. Nutr Metab (Lond). 2023;20(1):6. [26] LI M, VAN ESCH B, WAGENAAR GTM, et al. Pro- and anti-inflammatory effects of short chain fatty acids on immune and endothelial cells. Eur J Pharmacol. 2018; 831:52-59. [27] ZHU L, HUA F, DING W, et al. The correlation between the Th17/Treg cell balance and bone health. Immun Ageing. 2020;17:30. [28] YANG X, WANG Y, HAN X, et al. Effects of TGF-β1 on OPG/RANKL expression of cementoblasts and osteoblasts are similar without stress but different with mechanical compressive stress. ScientificWorldJournal. 2015;2015:718180. [29] SUN P, ZHANG C, HUANG Y, et al. Jiangu granule ameliorated OVX rats bone loss by modulating gut microbiota-SCFAs-Treg/Th17 axis. Biomed Pharmacother. 2022;150:112975. [30] GUO M, LIU H, YU Y, et al. Lactobacillus rhamnosus GG ameliorates osteoporosis in ovariectomized rats by regulating the Th17/Treg balance and gut microbiota structure. Gut Microbes. 2023;15(1):2190304. [31] LUCAS S, OMATA Y, HOFMANN J, et al. Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat Commun. 2018;9(1):55. [32] LI X, HAN Y, GUAN Y, et al. Aluminum induces osteoblast apoptosis through the oxidative stress-mediated JNK signaling pathway. Biol Trace Elem Res. 2012; 150(1-3):502-508. [33] 汪青,钱胤华,黄昊强,等.氧化应激在骨质疏松症与肩袖损伤共病机制中的研究进展[J].中国骨质疏松杂志,2023,29(5):776-780. [34] LORENZO D, GIANVINCENZO Z, CARLO LUCA R, et al. Oral-Gut Microbiota and Arthritis: Is There an Evidence-Based Axis? J Clin Med. 2019;8(10):0. doi: 10.3390/jcm8101753. [35] SUDO N, CHIDA Y, AIBA Y, et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. 2004;558(Pt 1):263-275. [36] COOPER MS. Sensitivity of bone to glucocorticoids. Clin Sci (Lond). 2004;107(2): 111-123. [37] ZHOU RX, ZHANG YW, CAO MM, et al. Linking the relation between gut microbiota and glucocorticoid-induced osteoporosis. J Bone Miner Metab. 2023; 41(2):145-162. [38] HONG S, CHA KH, PARK JH, et al. Cinnamic acid suppresses bone loss via induction of osteoblast differentiation with alteration of gut microbiota. J Nutr Biochem. 2022;101:108900. [39] XIAO L, ZHOU Y, BOKOLIYA S, et al. Bone loss is ameliorated by fecal microbiota transplantation through SCFA/GPR41/ IGF1 pathway in sickle cell disease mice. Sci Rep. 2022;12(1):20638. [40] YADAV VK, RYU JH, SUDA N, et al. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell. 2008;135(5):825-837. [41] XIAO HH, ZHU YX, LU L, et al. The Lignan-Rich Fraction from Sambucus williamsii Hance Exerts Bone Protective Effects via Altering Circulating Serotonin and Gut Microbiota in Rats. Nutrients. 2022;14(22):4718. [42] LI J Y, YU M, PAL S, et al. Parathyroid hormone-dependent bone formation requires butyrate production by intestinal microbiota. J Clin Invest. 2020;130(4): 1767-1781. [43] LI Z, HUANG J, WANG F, et al. Dual Targeting of Bile Acid Receptor-1 (TGR5) and Farnesoid X Receptor (FXR) Prevents Estrogen-Dependent Bone Loss in Mice. J Bone Miner Res. 2019;34(4):765-776. [44] WANG Q, WANG G, WANG B, et al. Activation of TGR5 promotes osteoblastic cell differentiation and mineralization. Biomed Pharmacother. 2018;108:1797-1803. [45] CHO SW, AN JH, PARK H, et al. Positive regulation of osteogenesis by bile acid through FXR. J Bone Miner Res. 2013;28(10):2109-2121. [46] WEI X, YANG X, HAN ZP, et al. Mesenchymal stem cells: a new trend for cell therapy. Acta Pharmacol Sin. 2013;34(6):747-754. [47] OZASA R, MATSUGAKI A, ISOBE Y, et al. Construction of human induced pluripotent stem cell-derived oriented bone matrix microstructure by using in vitro engineered anisotropic culture model. J Biomed Mater Res A. 2018;106(2): 360-369. [48] XING J, YING Y, MAO C, et al. Hypoxia induces senescence of bone marrow mesenchymal stem cells via altered gut microbiota. Nat Commun. 2018;9(1): 2020. [49] LIN H, LIU T, LI X, et al. The role of gut microbiota metabolite trimethylamine N-oxide in functional impairment of bone marrow mesenchymal stem cells in osteoporosis disease. Ann Transl Med. 2020;8(16):1009. [50] CHEN CY, RAO SS, YUE T, et al. Glucocorticoid-induced loss of beneficial gut bacterial extracellular vesicles is associated with the pathogenesis of osteonecrosis. Sci Adv. 2022;8(15):eabg8335. [51] LI W, LI T, TANG Z, et al. Taohong Siwu decoction promotes the process of fracture healing by activating the VEGF-FAK signal pathway and systemically regulating the gut microbiota. J Appl Microbiol. 2022;133(3):1363-1377. [52] QUEIPO-ORTUÑO MI, SEOANE LM, MURRI M, et al. Gut microbiota composition in male rat models under different nutritional status and physical activity and its association with serum leptin and ghrelin levels. PLoS One. 2013;8(5):e65465. [53] LAMBERT JE, MYSLICKI JP, BOMHOF MR, et al. Exercise training modifies gut microbiota in normal and diabetic mice. Appl Physiol Nutr Metab. 2015;40(7): 749-752. [54] LIU Z, LIU HY, ZHOU H, et al. Moderate-Intensity Exercise Affects Gut Microbiome Composition and Influences Cardiac Function in Myocardial Infarction Mice. Front Microbiol. 2017;8:1687. [55] WANG J, ZHANG Q, XIA J, et al. Moderate Treadmill Exercise Modulates Gut Microbiota and Improves Intestinal Barrier in High-Fat-Diet-Induced Obese Mice via the AMPK/CDX2 Signaling Pathway. Diabetes Metab Syndr Obes. 2022;15: 209-223. [56] ESTAKI M, PITHER J, BAUMEISTER P, et al. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome. 2016;4(1):42. [57] ALLEN JM, MAILING LJ, NIEMIRO GM, et al. Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans. Med Sci Sports Exerc. 2018;50(4):747-757. [58] BARTON W, PENNEY NC, CRONIN O, et al. The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level. Gut. 2018;67(4):625-633. [59] MOTIANI KK, COLLADO MC, ESKELINEN JJ, et al. Exercise Training Modulates Gut Microbiota Profile and Improves Endotoxemia. Med Sci Sports Exerc. 2020;52(1): 94-104. [60] WU J, OKA J, HIGUCHI M, et al. Cooperative effects of isoflavones and exercise on bone and lipid metabolism in postmenopausal Japanese women: a randomized placebo-controlled trial. Metabolism. 2006;55(4):423-433. [61] CASTRO AP, SILVA KKS, MEDEIROS CSA, et al. Effects of 12 weeks of resistance training on rat gut microbiota composition. J Exp Biol. 2021;224(12):242543. [62] 雷岱. HIIT和MICT对高脂膳食大鼠肠道菌群与TLR4蛋白的影响[D].石家庄:河北师范大学,2021. [63] DENOU E, MARCINKO K, SURETTE MG, et al. High-intensity exercise training increases the diversity and metabolic capacity of the mouse distal gut microbiota during diet-induced obesity. Am J Physiol Endocrinol Metab. 2016;310(11):E982-993. [64] MCKENNA CF, SALVADOR AF, HUGHES RL, et al. Higher protein intake during resistance training does not potentiate strength, but modulates gut microbiota, in middle-aged adults: a randomized control trial. Am J Physiol Endocrinol Metab. 2021;320(5):E900-E913. [65] DUPUIT M, RANCE M, MOREL C, et al. Effect of Concurrent Training on Body Composition and Gut Microbiota in Postmenopausal Women with Overweight or Obesity. Med Sci Sports Exerc. 2022;54(3):517-529. [66] RETTEDAL EA, CREE JME, ADAMS SE, et al. Short-term high-intensity interval training exercise does not affect gut bacterial community diversity or composition of lean and overweight men. Exp Physiol. 2020;105(8):1268-1279. [67] LEE CS, KIM BK, LEE IO, et al. Prevention of bone loss by using Lactobacillus-fermented milk products in a rat model of glucocorticoid-induced secondary osteoporosis. Int Dairy J. 2020;109:526-533. [68] LI K, LIU A, ZONG W, et al. Moderate exercise ameliorates osteoarthritis by reducing lipopolysaccharides from gut microbiota in mice. Saudi J Biol Sci. 2021; 28(1): 40-49. [69] 刘洋.高脂膳食和运动调控小鼠肠道菌群及LPS-TLR4/NF-κB信号通路在肥胖性骨性关节炎中的作用[D].上海:上海体育学院,2019. [70] 梁家琪,刘恒旭,阳金鑫,等.运动与肠道菌健康效益的关系[J].中国组织工程研究,2023,27(8):1292-1299. [71] MCCABE LR, IRWIN R, TEKALUR A, et al. Exercise prevents high fat diet-induced bone loss, marrow adiposity and dysbiosis in male mice. Bone. 2019;118:20-31. [72] YUAN FL, LI X, LU WG, et al. Regulatory T cells as a potent target for controlling bone loss. Biochem Biophys Res Commun. 2010;402(2):173176. [73] CHEN H, SHEN L, LIU Y, et al. Strength Exercise Confers Protection in Central Nervous System Autoimmunity by Altering the Gut Microbiota. Front Immunol. 2021;12:628629. [74] ZHANG YW, CAO MM, LI YJ, et al. Fecal microbiota transplantation as a promising treatment option for osteoporosis. J Bone Miner Metab. 2022;40(6):874-889. [75] HUANG WC, TUNG CL, YANG YSH, et al. Endurance exercise ameliorates Western diet-induced atherosclerosis through modulation of microbiota and its metabolites. Sci Rep. 2022;12(1):3612. [76] 吴彬,张勇,陈明亮,等.雌马酚对去卵巢后大鼠骨质疏松症的影响[J].第三军医大学学报,2015,37(3):256-260. [77] CORNISH J, CALLON KE, BAVA U, et al. Leptin directly regulates bone cell function in vitro and reduces bone fragility in vivo. J Endocrinol. 2002;175(2):405-415. [78] RIOS JL, BOMHOF MR, REIMER RA, et al. Protective effect of prebiotic and exercise intervention on knee health in a rat model of diet-induced obesity. Sci Rep. 2019;9(1):3893. [79] MEISSNER M, LOMBARDO E, HAVINGA R, et al. Voluntary wheel running increases bile acid as well as cholesterol excretion and decreases atherosclerosis in hypercholesterolemic mice. Atherosclerosis. 2011;218(2):323-329. [80] CHEN SEE JR, AMOS D, WRIGHT J, et al. Synergistic effects of exercise and catalase overexpression on gut microbiome. Environ Microbiol. 2022;24(9):4220-4235. [81] MUNUKKA E, AHTIAINEN JP, PUIGBó P, et al. Six-Week Endurance Exercise Alters Gut Metagenome That Is not Reflected in Systemic Metabolism in Over-weight Women. Front Microbiol. 2018;9:2323. [82] UDAY S, HÖGLER W. Nutritional Rickets and Osteomalacia in the Twenty-first Century: Revised Concepts, Public Health, and Prevention Strategies. Curr Osteoporos Rep. 2017;15(4):293-302. [83] 王培霞,张勤,周石仙,等.骨质疏松症营养干预研究进展[J].中国骨质疏松杂志,2023,29(3):409-412+443. [84] ILESANMI-OYELERE BL, ROY NC, KRUGER MC. Modulation of Bone and Joint Biomarkers, Gut Microbiota, and Inflammation Status by Synbiotic Supplementation and Weight-Bearing Exercise: Human Study Protocol for a Randomized Controlled Trial. JMIR Res Protoc. 2021;10(10):e30131. [85] LUO B, XIANG D, NIEMAN DC, et al. The effects of moderate exercise on chronic stress-induced intestinal barrier dysfunction and antimicrobial defense. Brain Behav Immun. 2014;39:99-106. [86] FU R, NIU R, ZHAO F, et al. Exercise alleviated intestinal damage and microbial disturbances in mice exposed to fluoride. Chemosphere. 2022;288:132658. [87] DONOHOE DR, GARGE N, ZHANG X, et al. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011; 13(5):517-526. |
| [1] | 郭苏童, 冯德宏, 郭 宇, 王 凌, 丁育健, 刘 仪, 钱正瑛, 李明洋. 正常与骨质疏松髋关节模型的建立及有限元分析[J]. 中国组织工程研究, 2024, 28(9): 1342-1346. |
| [2] | 吴 菁, 姚英策, 杨晓巍, 薛博士, 赵建斌, 杨 辰, 栾天峰, 周志鹏. 肌力训练与神经肌肉电刺激干预髌股关节痛患者下肢功能和生物力学的变化[J]. 中国组织工程研究, 2024, 28(9): 1365-1371. |
| [3] | 杨策凯, 蔡卓延, 陈 明, 刘 昊, 翁 汭, 崔健超, 张顺聪, 姚珍松. 绝经后女性椎旁肌退化与经皮穿刺椎体成形后再骨折的相关性[J]. 中国组织工程研究, 2024, 28(9): 1414-1419. |
| [4] | 娄 国, 张 艳, 付常喜. 内皮型一氧化氮合酶在运动预适应改善心肌缺血-再灌注损伤中的作用[J]. 中国组织工程研究, 2024, 28(8): 1283-1288. |
| [5] | 章晓云, 刘 桦, 柴 源, 陈 锋, 曾 浩, 高振罡, 黄有荣. 益肾固疏方干预老年性骨质疏松症患者骨代谢标志物的变化及临床疗效[J]. 中国组织工程研究, 2024, 28(8): 1155-1160. |
| [6] | 赵嘎日达, 任逸众, 韩长旭, 孔令跃, 贾岩波. 蒙药额尔敦-乌日勒修复骨关节炎大鼠模型的机制[J]. 中国组织工程研究, 2024, 28(8): 1193-1199. |
| [7] | 王 继, 张 敏, 李文博, 杨中亚, 张 龙. 有氧运动对2型糖尿病大鼠糖脂代谢、骨骼肌炎症和自噬的影响[J]. 中国组织工程研究, 2024, 28(8): 1200-1205. |
| [8] | 代越星, 郑利钦, 吴敏辉, 李志鸿, 李少彬, 郑德声, 林梓凌. 血管数量对小血管网计算流体力学的影响[J]. 中国组织工程研究, 2024, 28(8): 1206-1210. |
| [9] | 周邦瑜, 李 杰, 阮玉山, 耿福能, 李绍波. 美洲大蠊研粉干预脊髓半横断大鼠运动功能和自噬蛋白Beclin-1的表达[J]. 中国组织工程研究, 2024, 28(8): 1223-1228. |
| [10] | 阮 蓉, 娄旭佳, 金其贯, 章立冰, 徐 尚, 胡玉龙. 白藜芦醇可调控运动性疲劳大鼠的糖异生[J]. 中国组织工程研究, 2024, 28(8): 1229-1234. |
| [11] | 童奕博 , 李明辉. 骨质疏松性椎体骨折患者椎体成形后邻近椎体再发骨折的影响因素[J]. 中国组织工程研究, 2024, 28(8): 1241-1246. |
| [12] | 张 敏, 彭 婧, 张 强, 陈德旺. 有限元法分析老年骨质疏松患者L3/4椎板减压椎间融合的力学性能[J]. 中国组织工程研究, 2024, 28(6): 847-851. |
| [13] | 薛晓峰, 魏永康, 乔晓红, 杜玉勇, 牛建军, 任立新, 杨慧峰, 张治民, 郭 媛, 陈维毅. 股骨颈骨折空心钉内固定后股骨近端骨质疏松的有限元分析[J]. 中国组织工程研究, 2024, 28(6): 862-867. |
| [14] | 凯依塞尔•阿布都克力木, 麦麦提敏•阿卜力米提, 李 磊, 杨晓凯, 张玉坤, 刘 帅. 女性腰椎退行性病变患者腰椎CT值对骨质疏松症的诊断作用[J]. 中国组织工程研究, 2024, 28(6): 945-949. |
| [15] | 王力平, 连天星, 胡永荣, 杨红胜, 曾智谋, 刘 浩, 屈 波. 胸部CT椎体HU值在2型糖尿病骨质疏松症机会性筛查中的价值[J]. 中国组织工程研究, 2024, 28(6): 950-954. |
近年来,肠道菌群研究在炎症性肠病、肥胖、糖尿病和心血管疾病等代谢性疾病及相关领域被广泛关注[3]。大量研究表明,肠道菌群及其代谢物会通过各种信号通路参与骨质疏松的发生发展,骨质疏松患者与健康人群肠道菌群的组成和分布特征存在极大差异;此外,肠道菌群稳态失调会对机体菌群代谢、免疫炎症及内分泌等功能产生影响,继而影响骨代谢平衡[4]。研究发现,以肠道菌群为靶点,通过菌群定植、益生菌补充和运动干预等不同方式可延缓骨质疏松的发生[5]。而运动干预可通过调节对骨骼的机械应力作用、钙的吸收以及骨代谢信号通路表达等方式改善骨质流失,进而防治骨质疏松[6]。新近研究表明,运动调控肠道菌群能通过不同途径发挥预防和治疗骨质流失的作用,为运动防治骨质疏松提供了新视野。现阶段有关肠道菌群对骨质疏松作用机制的研究需要进一步探索和完善,且运动通过肠道菌群防治骨质疏松的相关研究较少。该文对运动介导肠道菌群调控骨质疏松进行深入分析,以期为改善骨质疏松相关研究提供参考。 中国组织工程研究杂志出版内容重点:组织构建;骨细胞;软骨细胞;细胞培养;成纤维细胞;血管内皮细胞;骨质疏松;组织工程
1.1.1 检索人及检索时间 由第一作者于2023年1-5月进行文献检索收集。
1.1.2 检索文献时限 检索1990年1月至2023年5月发表的相关文献。
1.1.3 检索数据库 CNKI和PubMed数据库。
1.1.4 检索词 中文检索词为“肠道菌群,肠道细菌,肠道菌群代谢物,骨代谢,骨质疏松和运动”,英文检索词为“Intestinal flora,intestinal bacteria,metabolites of intestinal flora,bone metabolism, osteoporosis,exercise”。
1.1.5 检索文献类型 综述、荟萃分析、期刊文章、学位论文等。
1.1.6 检索策略 输入关键词,以标题和摘要为检索条件进行检索。以PubMed数据库为例的检索策略,见图1。

1.2 资料筛选流程与筛选标准
1.2.1 纳入标准 肠道菌群与骨质疏松的相关研究;肠道菌群代谢物与骨质疏松的相关研究;运动与肠道菌群的相关研究;运动介导肠道菌群调控骨质疏松的相关研究。
1.2.2 排除标准 会议、讲座、重复性研究以及数据报告不完整或数据无法索取的研究。
1.3 文献质量评价 通过阅读标题、摘要及关键词对文献进行初步筛选,排除重复研究及与纳入标准无关的中英文文献,查阅全文最终保留87篇文献,包括中文文献9篇、英文文献78篇,见图2。

3.2 作者综述区别于他人他篇的特点 该综述从不同骨细胞角度总结了目前学者关于肠道菌群在骨质疏松中的作用以及运动调控肠道菌群改善骨质疏松的最新研究成果。首先,介绍了肠道菌群组成及其代谢物水平变化能在骨质疏松中发挥诊断作用;其次,重点介绍了肠道菌群对不同骨细胞的作用机制,肠道菌群失调诱导破骨细胞形成、加速成骨细胞凋亡和影响骨髓间充质干细胞;而调节肠道菌群稳态和上调短链脂肪酸水平能抑制破骨细胞分化、促进成骨细胞分化和促进骨髓间充质干细胞发育、抑制骨细胞凋亡并促进其向成骨细胞转化,防治骨质流失。最后,指出合理运动能调控肠道菌群能抑制骨细胞凋亡、抑制破骨细胞分化、促进成骨细胞分化和调节骨细胞营养代谢,进而发挥防治骨质疏松的潜在作用。此外,总结了不同运动形式防治骨质流失的作用差异,如:高强度自行车滚轮运动能调控肠道菌群、减轻氧化应激、抑制骨细胞凋亡;步行能介导内分泌调控肠道菌群对成骨细胞分化产生积极作用等。
3.3 综述的局限性 虽然该文从不同骨细胞角度系统综述了肠道菌群及其代谢物对骨质疏松的影响,并探讨运动介导肠道菌群调控骨质疏松的潜在作用,但现阶段关于肠道菌群调控不同骨细胞的深层机制以及运动介导肠道菌群调控骨代谢防治骨质流失的报道较为有限。运动调控肠道菌群受机体自身状态、饮食和锻炼方式等多种影响,运动对肠道菌群的影响仍需具体探索,因此在阐释运动介导肠道菌群对不同骨细胞作用与机制中存在一定的局限性,后续仍有待进一步深入研究。
3.4 综述的重要意义 文章从肠道菌群对破骨细胞、成骨细胞以及骨髓间充质干细胞等骨细胞的作用机制这个角度,阐释了肠道菌群在骨质疏松中的诊疗和通过运动调控肠道菌群进而改善骨质疏松的相关研究提供参考依据。运动通过调控肠道菌群能调节肠道菌群代谢物水平、改善肠屏障功能、抑制炎症反应、介导内分泌和调节骨免疫等不同方式进而抑制骨细胞凋亡、抑制破骨细胞分化、促进成骨细胞分化和调节骨细胞营养代谢等,重塑骨形成和骨重建等过程促进骨健康。随着对肠道菌群-运动-骨代谢的深入研究探讨,相信这些研究可在未来的骨质疏松防治中发挥关键作用。
3.5 课题组专家的建议 ①不同运动形式对肠道菌群的影响的具体生物学机制及差异性需进一步研究;②在确定特定肠道菌群在骨质疏松的诊断与治疗中应采用无菌对照等方式进行研究,以获得可靠数据;③目前,运动调控肠道菌群改善骨质疏松这一防治思路仍有待探讨,尚待深入研究提供可靠依据。 中国组织工程研究杂志出版内容重点:组织构建;骨细胞;软骨细胞;细胞培养;成纤维细胞;血管内皮细胞;骨质疏松;组织工程
 #br#
#br#
文题释义:
肠道菌群:生活在人体肠道内所有微生物的总和,其种类超过 1 000种,遍布于十二指肠、小肠和结肠,总数量约为 1014个,它们与宿主和谐共生形成稳态。运动介导肠道菌群在骨质疏松中的调控作用:运动可通过调节肠道菌群及其代谢物分泌,从而发挥抑制骨细胞凋亡、抑制破骨细胞分化、促进成骨细胞分化及调节骨细胞营养代谢等作用,进而在防治骨质疏松中发挥有益效应。
肠道菌群失调会加剧炎症反应和诱导氧化应激,从而促使骨细胞凋亡和成骨细胞分化,进而加剧骨质流失;调控肠道菌群能抑制破骨细胞分化、促进成骨细胞分化、抑制骨细胞凋亡以及调节骨髓间充质干细胞功能。合理运动有益于改善机体不同系统,其中部分作用机制源于运动对肠道菌群的调节,跑步机运动、橄榄球运动、自行车以及步行等有氧运动和抗阻运动均可重塑肠道菌群,增加肠道菌群多样性和丰度,而高强间歇运动对肠道菌群的影响却存在差异。运动介导肠道菌群具有改善骨代谢的潜在作用,运动能调控肠道菌群稳态、改善肠屏障功能、促进短链脂肪酸和胆汁酸分泌、下调血清脂多糖水平、降低氧化应激,进而抑制骨细胞凋亡、抑制破骨细胞分化、促进成骨细胞分化和调节骨细胞营养代谢,防治骨质流失。
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||