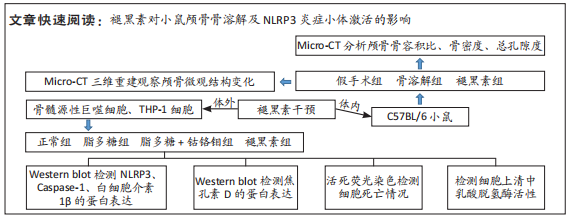
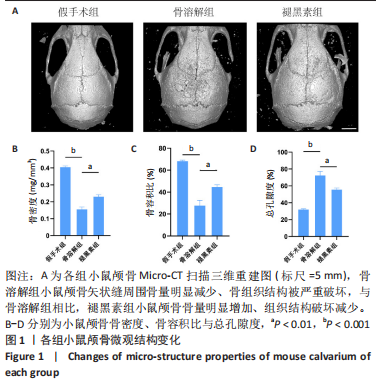
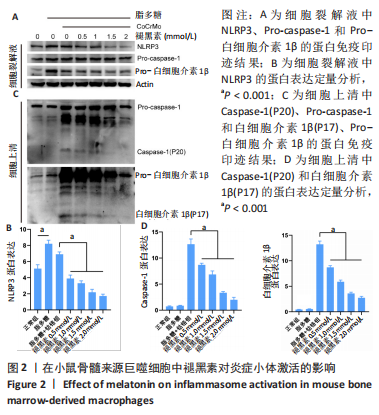
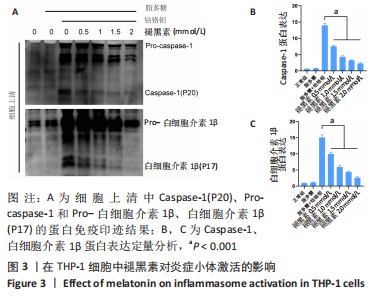
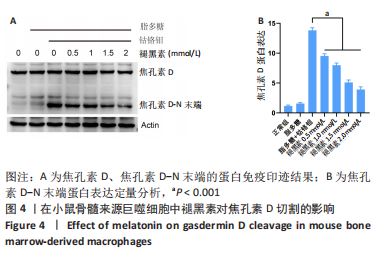
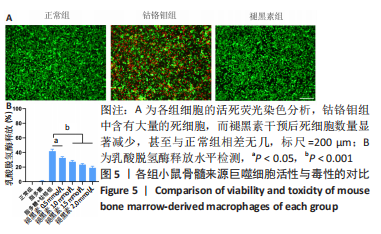
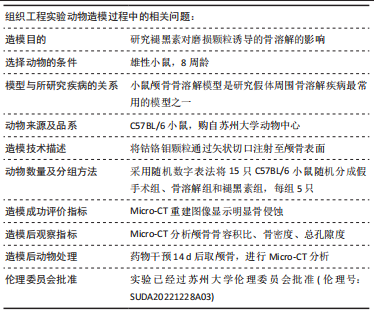
[1] ALHASAN H, TERKAWI MA, MATSUMAE G, et al. Inhibitory role of Annexin A1 in pathological bone resorption and therapeutic implications in periprosthetic osteolysis. Nat Commun. 2022;13(1):3919.
[2] GALLO J, GOODMAN SB, KONTTINEN YT, et al. Osteolysis around total knee arthroplasty: a review of pathogenetic mechanisms. Acta Biomater. 2013;9(9):8046-8058.
[3] INGHAM E, FISHER J. The role of macrophages in osteolysis of total joint replacement. Biomaterials. 2005;26(11):1271-1286.
[4] SUN KY, WU Y, XU J, et al. Niobium carbide (MXene) reduces UHMWPE particle-induced osteolysis. Bioact Mater. 2022;8:435-448.
[5] HODGES NA, SUSSMAN EM, STEGEMANN JP. Aseptic and septic prosthetic joint loosening: Impact of biomaterial wear on immune cell function, inflammation, and infection. Biomaterials. 2021;278:121127.
[6] COLL RC, SCHRODER K, PELEGRIN P. NLRP3 and pyroptosis blockers for treating inflammatory diseases. Trends Pharmacol Sci. 2022;43(8):653-668.
[7] FARIA SS, COSTANTINI S, DE LIMA VCC, et al. NLRP3 inflammasome-mediated cytokine production and pyroptosis cell death in breast cancer. J Biomed Sci. 2021;28(1): 26.
[8] HUANG Y, XU W, ZHOU R. NLRP3 inflammasome activation and cell death. Cell Mol Immunol. 2021;18(9):2114-2127.
[9] LI X, ZHANG X, XIA J, et al. Macrophage HIF-2alpha suppresses NLRP3 inflammasome activation and alleviates insulin resistance. Cell Rep. 2021;36(8):109607.
[10] SWANSON KV, DENG M, TING JP. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19(8):477-489.
[11] MASTERS SL, DUNNE A, SUBRAMANIAN SL, et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1beta in type 2 diabetes. Nat Immunol. 2010;11(10):897-904.
[12] CAICEDO MS, DESAI R, MCALLISTER K, et al. Soluble and particulate Co-Cr-Mo alloy implant metals activate the inflammasome danger signaling pathway in human macrophages: a novel mechanism for implant debris reactivity. J Orthop Res. 2009;27(7):847-854.
[13] BAI D, DU J, BU X, et al. ALDOA maintains NLRP3 inflammasome activation by controlling AMPK activation. Autophagy. 2022;18(7):1673-1693.
[14] SHARMA BR, KANNEGANTI TD. NLRP3 inflammasome in cancer and metabolic diseases. Nat Immunol. 2021;22(5):550-559.
[15] YIN J, YIN Z, LAI P, et al. Pyroptosis in Periprosthetic Osteolysis. Biomolecules. 2022;12(12): 1733.
[16] LI Y, LING J, JIANG Q. Inflammasomes in Alveolar Bone Loss. Front Immunol. 2021;12: 691013.
[17] JAMSEN E, PAJARINEN J, KOURI VP, et al. Tumor necrosis factor primes and metal particles activate the NLRP3 inflammasome in human primary macrophages. Acta Biomater. 2020; 108:347-357.
[18] CAICEDO MS, SAMELKO L, MCALLISTER K, et al. Increasing both CoCrMo-alloy particle size and surface irregularity induces increased macrophage inflammasome activation in vitro potentially through lysosomal destabilization mechanisms. J Orthop Res. 2013;31(10): 1633-1642.
[19] WU Y, HE F, ZHANG C, et al. Melatonin alleviates titanium nanoparticles induced osteolysis via activation of butyrate/GPR109A signaling pathway. J Nanobiotechnol. 2021;19(1):170.
[20] SHEN S, LIAO Q, WONG YK, et al. The role of melatonin in the treatment of type 2 diabetes mellitus and Alzheimer’s disease. Int J Biol Sci. 2022;18(3):983-994.
[21] LIN TH, TAMAKI Y, PAJARINEN J, et al. Chronic inflammation in biomaterial-induced periprosthetic osteolysis: NF-kappaB as a therapeutic target. Acta Biomater. 2014;10(1):1-10.
[22] XU Y, SANG W, ZHONG Y, et al. CoCrMo-Nanoparticles induced peri-implant osteolysis by promoting osteoblast ferroptosis via regulating Nrf2-ARE signalling pathway. Cell Prolif. 2021;54(12):e13142.
[23] EGER M, HIRAM-BAB S, LIRON T, et al. Mechanism and Prevention of Titanium Particle-Induced Inflammation and Osteolysis. Front Immunol. 2018;9:2963.
[24] ZHENG K, BAI J, LI N, et al. Protective effects of sirtuin 3 on titanium particle-induced osteogenic inhibition by regulating the NLRP3 inflammasome via the GSK-3beta/beta-catenin signalling pathway. Bioact Mater. 2021;6(10):3343-3357.
[25] XUE S, XU Y, XU S, et al. Mitophagy impairment mediates the pathogenesis of CoCrMo particle-induced osteolysis via NLRP3/caspase-1/GSDMD-dependent pyroptosis in macrophages. Chem Eng J. 2022;435:135115-135115.
[26] WU Y, TENG Y, ZHANG C, et al. The ketone body beta-hydroxybutyrate alleviates CoCrMo alloy particles induced osteolysis by regulating NLRP3 inflammasome and osteoclast differentiation. J Nanobiotechnol. 2022;20(1):120.
[27] HOOFTMAN A, ANGIARI S, HESTER S, et al. The Immunomodulatory Metabolite Itaconate Modifies NLRP3 and Inhibits Inflammasome Activation. Cell Metab. 2020;32(3):468-478.e7.
[28] WU YL, ZHANG CH, TENG Y, et al. Propionate and butyrate attenuate macrophage pyroptosis and osteoclastogenesis induced by CoCrMo alloy particles. Mil Med Res. 2022; 9(1):46.
[29] YIN J, LI Y, HAN H, et al. Melatonin reprogramming of gut microbiota improves lipid dysmetabolism in high-fat diet-fed mice. J Pineal Res. 2018;65(4):e12524.
[30] CHEN J, XIA H, ZHANG L, et al. Protective effects of melatonin on sepsis-induced liver injury and dysregulation of gluconeogenesis in rats through activating SIRT1/STAT3 pathway. Biomed Pharmacother. 2019;117:109150.
[31] XU D, LIU L, ZHAO Y, et al. Melatonin protects mouse testes from palmitic acid-induced lipotoxicity by attenuating oxidative stress and DNA damage in a SIRT1-dependent manner. J Pineal Res. 2020;69(4):e12690.
[32] SU SC, HSIEH MJ, YANG WE, et al. Cancer metastasis: Mechanisms of inhibition by melatonin. J Pineal Res. 2017;62(1). doi: 10.1111/jpi.12370
[33] CHO JH, BHUTANI S, KIM CH, et al. Anti-inflammatory effects of melatonin: A systematic review and meta-analysis of clinical trials. Brain Behav Immun. 2021;93:245-253. |