中国组织工程研究 ›› 2020, Vol. 24 ›› Issue (16): 2572-2577.doi: 10.3969/j.issn.2095-4344.1889
• 材料生物相容性 material biocompatibility • 上一篇 下一篇
环保型生物组织样本制备套装在HER2蛋白2+浸润性乳腺癌荧光原位杂交检测中的应用
邱晓阳,王媛媛,刘春鹏,陈洪才,吴 璇,詹晓芬
- 汕头市中心医院病理科,广东省汕头市 515031
Application of environment-friendly bio-tissue sample preparation kit in fluorescence in situ hybridization detection of HER2 protein 2-positive invasive breast cancer
Qiu Xiaoyang, Wang Yuanyuan, Liu Chunpeng, Chen Hongcai, Wu Xuan, Zhan Xiaofen
- Department of Pathology, Shantou Central Hospital, Shantou 515031, Guangdong Province, China
摘要:
文题释义:
环保型生物组织样本制备套装:无醛、无苯,用于组织样本制备过程中对组织样本的固定、脱水和透明处理,其中固定液是利用无水乙醇、甲醇等化学试剂的蛋白质凝固作用,终止或减少分解酶的作用,防止自溶,保存组织、细胞的离体前结构状态;脱水液是利用能够与水互溶的有机溶液,将已固定和水洗过的组织中的水分彻底驱除;透明液是采用既能与脱水剂互溶,又能作为石蜡溶媒的有机溶剂,使浸蜡过程中石蜡渗入组织中。由于透明剂作用之后其折射指数与组织蛋白折射指数接近,组织显示出半透明状态,因此通常又称此过程为透明。
HER2蛋白:定位于染色体17q12-21.32上,编码相对分子质量为185 000的跨膜受体样蛋白,具有酪氨酸激酶活性。检测方法有免疫组织化学、荧光原位杂交等。HER2是指重要的乳腺癌及胃癌预后判断因子,HER2阳性(过表达或扩增)的乳腺癌/胃癌,其临床特点和生物学行为有特殊表现,治疗模式也与其他类型的乳腺癌/胃癌有很大的区别。目前已有针对该蛋白过度表达的药物——注射用曲妥珠单抗。
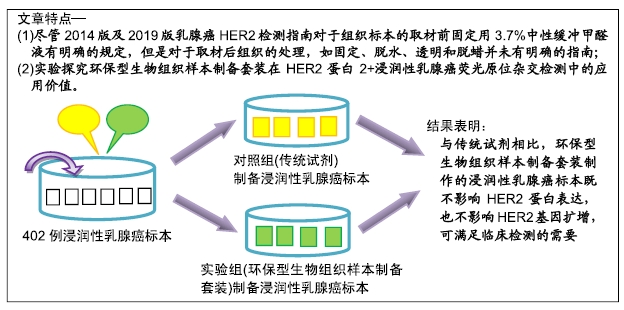
背景:HER2状态评估是浸润性乳腺癌治疗及预后重要的生物学指标。固定、脱水、透明和脱蜡等组织前期处理是制作病理石蜡切片后进行HER2蛋白和基因检测的必备程序,也是影响免疫组织化学和荧光原位杂交的重要因素。
目的:探究环保型生物组织样本制备套装在HER2蛋白2+浸润性乳腺癌荧光原位杂交检测中的应用价值。
方法:收集2015年1月至2019年3月汕头市中心医院送检的402例浸润性乳腺癌标本,同一标本对半剖开,使用随机数字表随机分为2组,对照组采用传统试剂甲醛固定-乙醇脱水-二甲苯透明脱蜡进行组织前期处理,制作石蜡切片;实验组采用环保型生物组织样本制备套装(含环保型固定液、脱水液、透明液、脱蜡液)制作切片。采用免疫组织化学法检测两组标本的HER2蛋白表达,进一步对HER2蛋白结果为2+的131例浸润性乳腺癌标本使用荧光原位杂交法检测HER2基因扩增。
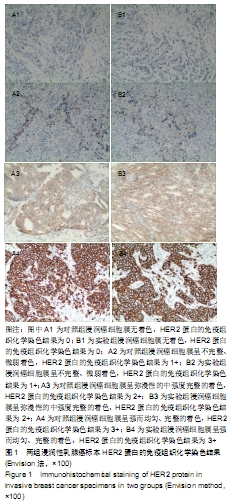
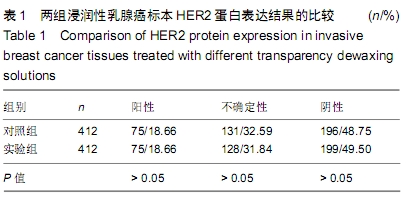
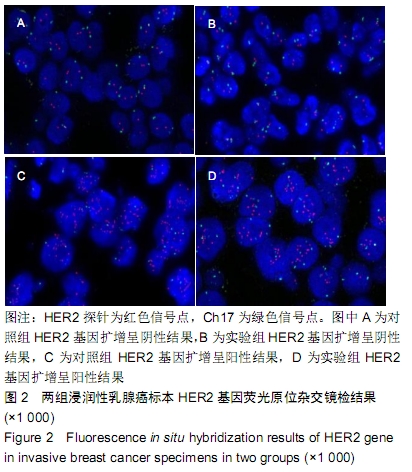
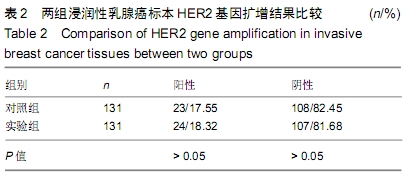
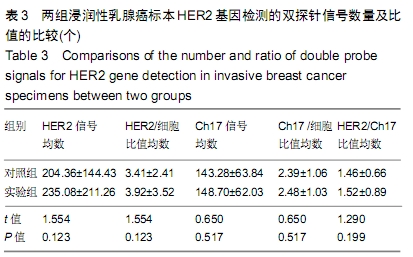
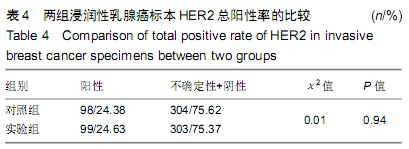
结果与结论:①两组HER2蛋白表达均为特异性的细胞膜着色、细胞定位正确;②两组HER2蛋白阳性率、不确定性率、阴性率比较差异均无显著性意义(P > 0.05),两组HER2蛋白表达符合率为99.00%;③两组HER2基因均背景清晰,HER2和Ch17双探针信号清晰可见,无交差反应、双探针信号精准定位于癌细胞核内;④两组HER2基因均杂交成功,两组杂交成功细胞数量比较差异无显著性意义(P > 0.05);⑤两组HER2基因扩增阳性率、阴性率比较差异均无显著性意义(P > 0.05),两组HER2基因扩增符合率为97.71%;⑥两组HER2基因信号均数、HER2/细胞比值均数、Ch17信号均数、Ch17 /细胞比值均数、HER2/ Ch17比值均数比较差异均无显著性意义(P > 0.05);⑦两组HER2总阳性率比较差异均无显著性意义(P > 0.05);⑧结果表明与传统试剂相比,环保型生物组织样本制备套装制作的浸润性乳腺癌标本既不影响HER2蛋白的表达,也不影响HER2基因扩增,可满足临床检测的需要。
ORCID: 0000-0003-0730-5467(邱晓阳)
中国组织工程研究杂志出版内容重点:生物材料;骨生物材料; 口腔生物材料; 纳米材料; 缓释材料; 材料相容性;组织工程
中图分类号: