中国组织工程研究 ›› 2019, Vol. 23 ›› Issue (26): 4136-4141.doi: 10.3969/j.issn.2095-4344.1349
• 组织工程口腔材料 tissue-engineered oral materials • 上一篇 下一篇
比较3种磷酸铵盐溶液制备碳酸磷灰石材料的理化性能
林 欣1,侯 敏2
- 天津市口腔医院,1口腔修复科,2整形外科,天津市 300041
Physical and chemical properties of carbonate apatite monolith treated by three ammonium phosphate solutions
Lin Xin1, Hou Min2
- 1Department of Prothodontics, 2Department of Orthographic Surgery, Tianjin Stomatological Hospital, Tianjin 300041, China
摘要:
文章快速阅读:
.jpg)
文题释义:
碳酸钙:作为骨代替材料已被研究多年,珊瑚作为其中的代表材料之一,主要以文石(aragonite)的形式存在,在高温条件下,热力学上相对不稳定的文石可变为较稳定的方解石。有研究发现将珊瑚碳酸钙放入磷酸盐溶液中进行水热处理可使其转变成珊瑚化羟基磷灰石,并成功应用于整形外科、口腔颌面外科临床。但由于珊瑚来源数量有限,并且很难获取医疗用途所需的高纯度的文石,因此无法得以广泛推广。
碳酸磷灰石:人体自然骨中磷灰石矿物主要是非化学计量的磷灰石晶体,与理论上的磷灰石成分Ca10(PO4)6(OH)2不同,还含有少量CO32-与Mg2+、Fe2+、Na+、HPO42-、F-、Cl-等微量元素。因此,骨成分的更适合的公式应为(Ca,X)10(PO4,CO3,Y)6(OH,Z)2,X替代阳离子,Y与Z替代阴离子,被称作碳酸磷灰石。
背景:课题组前期研究通过氢氧化钙的碳酸化处理制备了方解石团块,并在低温磷酸二氢铵盐溶液中处理合成了碳酸磷灰石团块,其具有良好的理化性能,但存在碳酸含量偏低等不足,考虑与磷酸二氢铵盐溶液较低的pH值有关。
目的:比较不同pH值磷酸铵盐溶液处理对方解石形成碳酸磷灰石骨替代材料理化性能的影响,寻找出在低温下制备出具有优良性能碳酸磷灰石团块的最理想磷酸铵盐溶液。
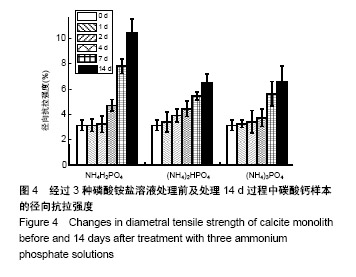
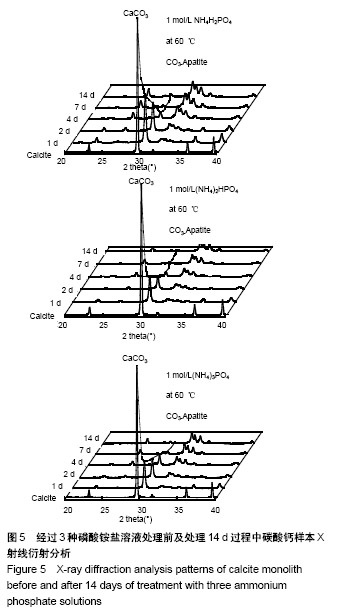
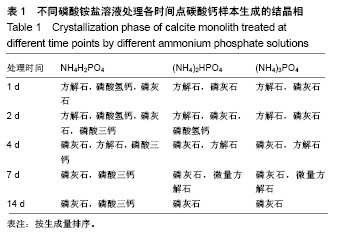
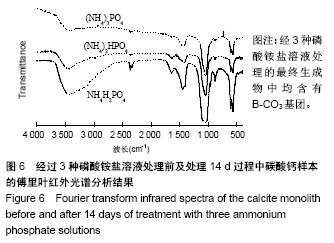
方法:将团块状氢氧化钙进行碳酸化处理生成方解石,分3组处理,分别浸泡于磷酸二氢铵盐、磷酸氢二铵盐、磷酸三铵盐溶液中(浓度均为1 mol/L,温度均为60 ℃)。浸泡1,4,7,14 d后,分别进行径向抗拉强度测试、X射线衍射分析、傅里叶变换红外线分析、扫描电镜观察及碳酸含量、钙磷比等理化性能检测。
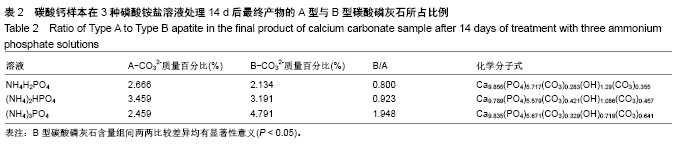
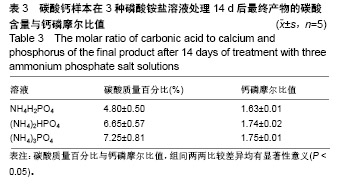
结果与结论:①X射线衍射、傅里叶变换红外线、扫描电镜、径向抗拉强度结果显示:经过3种磷酸铵盐溶液处理14 d后,CaCO3均已完全转化成为碳酸磷灰石,经NH4H2PO4水溶液处理的反应最快,最终样本达到最高的机械强度(10 MPa),但碳酸含量相对最低(4.80±0.5)%;经(NH4)2HPO4与(NH4)3PO4水溶液处理的最终样本径向抗拉强度值均约为6 MPa,可满足低负重部位骨缺损重建材料的机械强度要求;②化学分析结果显示:(NH4)3PO4水溶液处理最终样本的碳酸含量最高(7.25±0.81)%,与骨极其接近。并且B型碳酸磷灰石含量最高;③结果显示:3种磷酸铵盐溶液均可合成具有足够强度的低结晶性碳酸磷灰石团块,比较之下经(NH4)3PO4水溶液进行处理的最终产物最为理想。
中图分类号:


.jpg)