| [1]Arrington ED,Smith WJ,Chambers HG,et al. Complications of iliac crest bone graft harvesting.Clin Orthop Relat Res.1996;329:300-309.
[2]Urist MR.Bone Formation by autoinduction. Science. 1965;150:893-899.
[3]Celeste AJ,Iannazzi JA,Taylor RC,et al.Identification of transforming growth factor beta family members present in bone-inductive protein purified from bovine bone.Proc Natl Acad Sci USA. 1990;87: 9843-9847.
[4]Furlan AD,Pennick V,Bombardier C,et al.2009 updated method guidelines for systematic reviews in the Cochrane Back Review Group.Spine (Phila Pa 1976). 2009;34:1929-1941.
[5]Higgins JPT,Green S.Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0[updated March 2011].The Cochrane Collaboration.2011.
[6]DerSimonian R,Laird N. Meta-analysis in clinical trials. Control Clin Trials.1986;7:177-188.
[7]Egger M,Smith GD,Schneider M,et al.Bias in meta-analysis detected by a simple, graphical test. BMJ.1997;315(7109):629-634.
[8]Begg CB,Mazumdar M.Operating characteristics of a rank correlation test for publication bias.Biometrics. 1994;50(4):1088-1101.
[9]Egger M,Smith GD.Bias in location and selection of studies.BMJ.1998;316:61-66.
[10]Duval S,Tweedie R.Trim and fill: a simple funnel-plot based method of testing and adjusting for publication bias in meta-analysis.Biometrics. 2000;56(2): 455-463.
[11]Sutton AJ,Duval SJ,Tweedie RL,et al.Empirical assessment of effect of publication bias on meta-analyses.BMJ.2000;320(7249):1574-1577.
[12]Boden SD,Zdeblick TA,Sandhu HS,et al.The use of rhBMP-2 in interbody fusion cages. Definitive evidence of osteoinduction in humans: a preliminary report. Spine(Phila Pa 1976).2000;25(3):376-381.
[13]Boden SD,Kang J,Sandhu H,et al.Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial:2002 Volvo Award in clinical studies.Spine(Phila Pa 1976). 2002; 27(23):2662-2673.
[14]Burkus JK,Gornet MF,Dickman CA,et al.Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages.J Spinal Disord Tech. 2002;15:337-349.
[15]Burkus JK,Transfeldt EE,Kitchel SH,et al.Clinical and radiographic outcomes of anterior lumbar interbody fusion using recombinant human bone morphogenetic protein-2.Spine(Phila Pa 1976). 2002;27:2396-2408.
[16]Burkus JK,Dorchak JD,Sanders DL.Radiographic assessment of interbody fusion using recombinant human bone morphogenetic protein type 2.Spine(Phila Pa 1976).2003;28:372-377.
[17]Haid RW Jr,Branch CL Jr,Alexander JT,et al.Posterior lumbar interbody fusion using recombinant human bone morphogenetic protein type 2 with cylindrical interbody cages.Spine J.2004;4(5):527-538.
[18]Glassman SD,Dimar JR,Carreon LY,et al.Initial fusion rates with recombinant human bone morphogenetic protein-2/compression resistant matrix and a hydroxyapatite and tricalcium phosphate/collagen carrier in posterolateral spinal fusion.Spine(Phila Pa 1976).2005;30(15):1694-1698.
[19]Burkus JK,Sandhu HS,Gornet MF,et al.Use of rhBMP-2 in combination with structural cortical allografts: clinical and radiographic outcomes in anterior lumbar spinal surgery.J Bone Joint Surg Am. 2006;87(6):1205-1212.
[20]Dimar JR,Glassman SD,Burkus KJ,et al.Clinical outcomes and fusion success at 2 years of single-level instrumented posterolateral fusions with recombinant human bone morphogenetic protein-2/compression resistant matrix versus iliac crest bone graft.Spine (Phila Pa 1976).2006;31(22):2534-2539.
[21]Glassman SD,Carreon LY,Djurasovic M,et al. RhBMP-2 versus iliac crest bone graft for lumbar spine fusion: a randomized, controlled trial in patients over 60 years of age.Spine(Phila Pa 1976). 2008;33(26):2843-2849.
[22]Dimar JR 2nd,Glassman SD,Burkus JK,et al.Clinical and radiographic analysis of an optimized rhBMP-2 formulation as an autograft replacement in posterolateral lumbar spine arthrodesis.J Bone Joint Surg Am.2009;91(6):1377-1386.
[23]Dawson E,Bae HW,Burkus JK,et al.Recombinant human bone morphogenetic protein-2 on an absorbable collagen sponge with an osteoconductive bulking agent in posterolateral arthrodesis with instrumentation.A prospective randomized trial.J Bone Joint Surg Am.2009;91(7):1604-1613.
[24]Michielsen J,Sys J,Rigaux A,et al.The effect of recombinant human bone morphogenetic protein-2 in single-level posteriorlumbar interbody arthrodesis.J Bone Joint Surg Am.2013;95(10):873-880.
[25]Hurlbert RJ,Alexander D,Bailey S,et al.rhBMP-2 for posterolateral instrumented lumbar fusion: a multicenter prospective randomized controlled trial. Spine(Phila Pa 1976).2013;38:2139-2148.
[26]An H,Boden SD,Kang J,et al.Summary statement: emerging techniques for treatment of degenerative lumbar disc disease. Spine(Phila Pa 1976).2003;28(15 Suppl):S24-S25.
[27]Poynton AR,Lane JM.Safety profile for the clinical use of bone morphogenetic proteins in the spine. Spine (Phila Pa 1976).2002;27:S40-48.
[28]Benglis D,Wang MY,Levi AD.A comprehensive review of the safety profile of bone morphogenetic protein in spine surgery.Neurosurgery.2008;62(5 Suppl 2): ONS423-431.
[29]Carragee EJ,Hurwitz EL,Weiner BK.A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned.Spine J.2011;11(6):471-491.
[30]Mussano F,Ciccone G,Ceccarelli M,et al.Bone morphogenetic proteins and bone defects: a systematic review.Spine(Phila Pa 1976). 2007;32:824-830.
[31]Papakostidis C,Kontakis G,Bhandari M,et al.Efficacy of autologous iliac crest bone graft and bone morphogenetic proteins for posterolateral fusion of lumbar spine: a meta-analysis of the results. Spine(Phila Pa 1976). 2008;33(19):E680-692.
[32]Agarwal R,Williams K,Umscheid CA,et al. Osteoinductive bone graft substitutes for lumbar fusion: a systematic review.J Neurosurg Spine.2009; 11(11): 729-740.
[33]Donell S,Garrison K,Shemilt I,et al.Bone Morphogenetic Protein-2 for Spinal Fusion: Meta-analysis of Randomised Controlled Trials.UEA Website. Available:http://www.uea.ac.uk/~wo158 /BMP-2%20for%20SF%20-%20Meta-analysis%20of%20RCTs.pdf. Accessed Dec 12,2013.
[34]Blumenthal SL,Gill K.Can lumbar spine radiographs accurately determine fusion in postoperative patients? Correlation of routine radiographs with a second surgical look at lumbar fusions.Spine(Phila Pa 1976). 1993;18(9):1186-1189.
[35]Mroz TE,Wang JC,Hashimoto R,et al.Complications related to osteobiologics use in spine surgery: a systematic review.Spine(Phila Pa 1976). 2010;35(20): S86-104.
[36]Fu R,Selph S,McDonagh M,et al.Effectiveness and harms of recombinant human bone morphogenetic protein-2 in spine fusion: a systematic review and meta-analysis.Ann Intern Med.2013;158(12):890-902.
[37]Simmonds MC,Brown JV,Heirs MK,et al.Safety and effectiveness of recombinant human bone morphogenetic protein-2 for spinal fusion: a meta-analysis of individual-participant data.Ann Intern Med.2013;158(12):877-889.
[38]Carragee EJ,Hurwitz EL,Weiner BK.A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned.Spine J.2011;11: 471-491.
[39]Yale School of Medicine. Available: http://medicine.yale.edu/core/projects/yodap/datasharing/ medtronic/463_158787_YorkrhBMP2FinalReport. pdf.Accessed Dec 12,2013. |
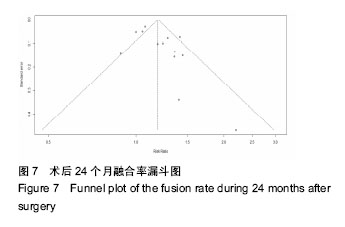
.jpg)

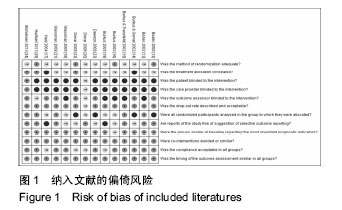
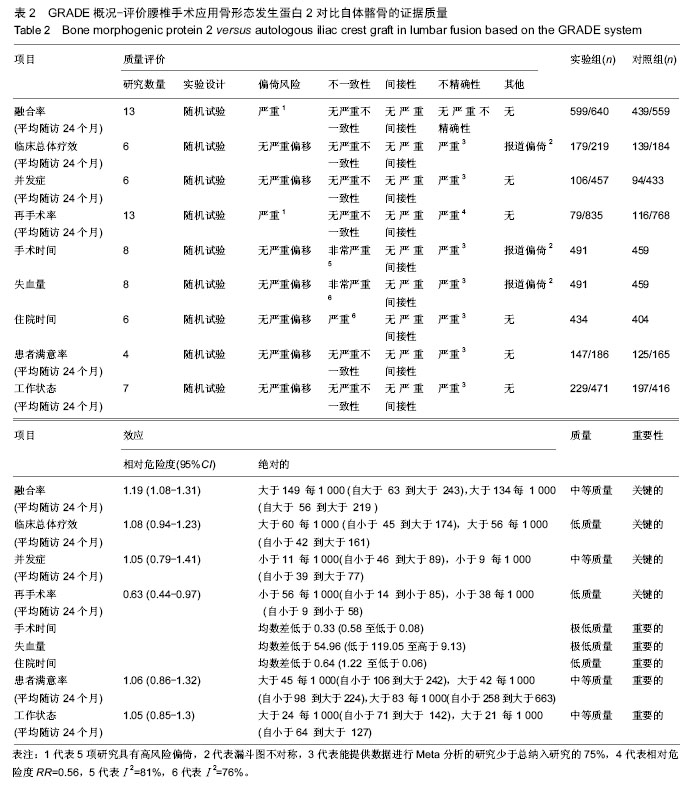
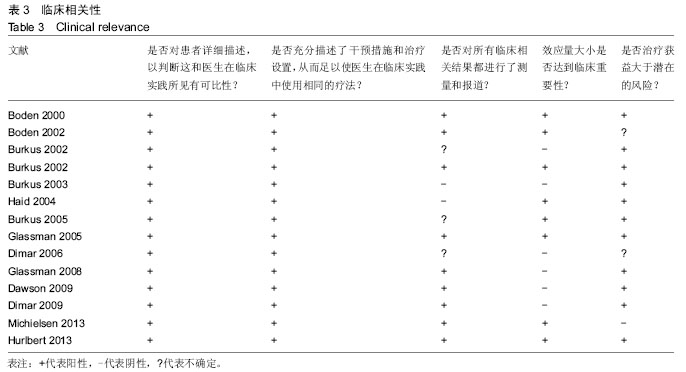
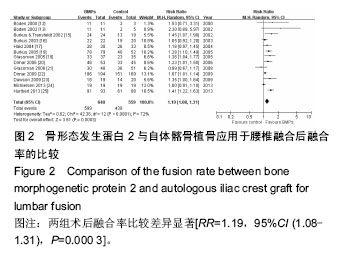
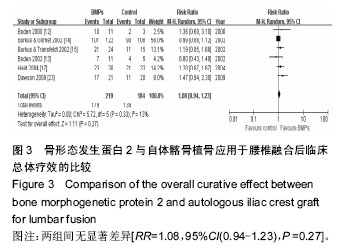
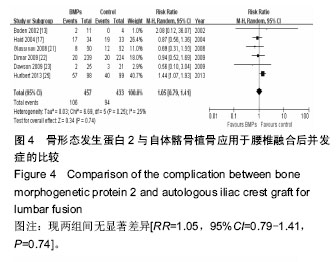
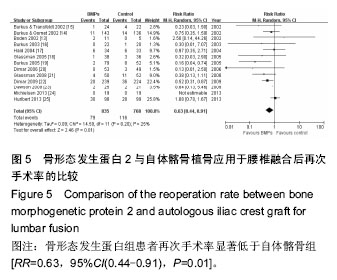
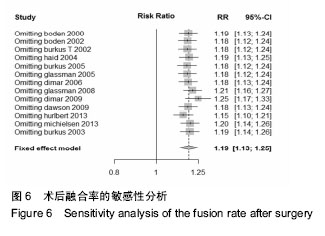
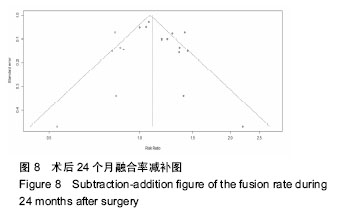
.jpg)