| [1] Spagnoli A.Mesenchymal stem cells and fracture healing.Orthopedics.2008;31(9):855-856.[2] Knight MN,Hankenson KD.Mesenchymal Stem Cells in Bone Regeneration.Adv Wound Care. 2013;2(6): 306-316.[3] Wang XL,Xie XH,Zhang G,et al.Exogenous phytoestrogenic molecule icaritin incorporated into a porous scaffold for enhancing bone defect repair.J Orthop Res.2013;31(1):164-172.[4] Chen DF,Zeng HP,Du SH,et al.Extracts from Plastrum testudinis promote proliferation of rat bone-marrow- derived mesenchymal stem cells.Cell Proliferat. 2007; 40(2):196-212.[5] Kang JW,Park KD,Choi Y,et al.Biodistribution and in vivo efficacy of genetically modified human mesenchymal stem cells systemically transplanted into a mouse bone fracture model.Arch Pharma Res. 2013; 36(8):1013-1022.[6] Choudhery MS,Badowski M,Muise A,et al.Donor age negatively impacts adipose tissue-derived mesenchymal stem cell expansion and differentiation.J Transl Med.2014;12:8.[7] Liu X,Zhou C,Li Y,et al.SDF-1 promotes endochondral bone repair during fracture healing at the traumatic brain injury condition.PloS One. 2013; 8(1):e54077.[8] Jadlowiec J,Koch H,Zhang X,et al.Phosphophoryn regulates the gene expression and differentiation of NIH3T3,MC3T3-E1,and human mesenchymal stem cells via the integrin/MAPK signaling pathway.J Biol Chem.2014;279(51):53323-53330.[9] Wu AC,Morrison NA,Kelly WL,Forwood MR.MCP-1 expression is specifically regulated during activation of skeletal repair and remodeling.Calcified Tissue Int. 2013; 92(6):566-575.[10] 王玉龙,王明贵,贺小兵,等.自体骨髓干细胞移植治疗胫骨骨折骨不连[J].局解手术学杂志, 2014,23(2): 131-134.[11] 段小军,杨柳,周跃,等.增强型绿色荧光蛋白标记技术对骨折后骨髓间充质干细胞的迁移示踪[J].中国修复重建外科杂志,2006,6(2):102-106.[12] Coipeau P,Rosset P,Langonne A,et al.Impaired differentiation potential of human trabecular bone mesenchymal stromal cells from elderly patients. Cytotherapy.2009;11(5):584-594.[13] Qiu W,Chen L,Kassem M.Activation of non-canonical Wnt/JNK pathway by Wnt3a is associated with differentiation fate determination of human bone marrow stromal(mesenchymal)stem cells.Biochem Biophys Res Commun. 2011;413(1): 98-104.[14] Nusse R.Wnt signaling in disease and in development. Cell Res.2015;5(15):28-32.[15] Wodarz A,Nusse R.Mechanisms of Wnt signaling in development.Cell Dev Biol.2008;14:59-88.[16] Bejsovec A.Wnt signaling:An embarrassment of receptors.Curr Biol.2000;10:R919-R922.[17] Kawano Y,Kypta R.Secreted antagonists of the Wnt signaling pathway.J Cell Sci.2003;116:2627-2634.[18] Qiu W,Andersen TE,Bollerslev J,et al.Patients with high bone mass phenotype exhibit enhanced osteoblast differentiation and inhibition of adipogenesis of human mesenchymal stem cells.J Bone Miner Res. 2007;22(11):1720-1731.[19] Day TF,Guo X,Garrett-Beal L,et al.Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis.Dev Cell.2015;8(5):739-50.[20] Savvidou OD,Mavrogenis AF,Sakellariou V,et al.Salvage of failed total hip arthroplasty with proximal femoral replacement.Orthopedics 2014;37(10):691-698.[21] Baghdadi YM,Jacobson JA,Duquin TR,et al.The outcome of total elbow arthroplasty in juvenile idiopathic arthritis(juvenile rheumatoid arthritis) patients.J Shoulder Elbow Surg. 2014; 23(9): 1374-1380.[22] Wyss T,Kägi P,Mayrhofer P,et al.Five-year results of the uncemented RM pressfit cup clinical evaluation and migration measurements by EBRA.J Arthroplasty. 2013;28(8):1291-1296.[23] Boland GM,Perkins G,Hall DJ,et al.Wnt3a promotes proliferation and suppresses osteogenic differentiation of adult human mesenchmal stem cells.Cell Biochem. 2014;93(6):1210-1230.[24] De Boer J,Wang HJ,Van Blitterswijk C.Effects of Wnt signaling on proliferation and differentiation of human mesenchymal stem cells.Tissue Eng. 2014;10(3-4): 393-401.[25] De Boer J,Siddappa R,Gaspar C,et al.Wnt signaling inhibits osetogenic differentiation of human mesenchymal stem cells.Bone.2014;34(5):818-826.[26] Etheridge SL,Spencer GJ,Heath DJ,et al.Expression profilling and functional analysis of wnt signaling mechanisms in mesenchymal stem cells.Stem Cells. 2014;22(5):849-860.[27] Chen YL,Lin T,Liu A,et al.Does hydroxyapatite coating have no advantage over porous coating in primary total hip arthroplasty?A meta-analysis.J Orthop Surg Res. 2015;10(1):21.[28] Zanotti B,Zingaretti N,Almesberger D,et al.Enhancing dermal and bone regeneration in calvarial defect surgery.Indian J Plast Surg.2014;47(3):325-332.[29] Djouad F,Bouffi C,Ghannam S,et al.Mesenchymal stem cells:innovative therapeutic tools for rheumatic diseases.Nat Rev Rheumatol.2009;5(7):392-399.[30] Bakhtina A,Tohfafarosh M,Lichtler A,et al. Characterization and differentiation potential of rabbit mesenchymal stem cells for translational regenerative medicine.In Vitro Cell Dev Biol Anim. 2014;50(3): 251-260.[31] Manuyakorn W,Howarth PH,Holgate ST.Airway remodelling in asthma and novel therapy.Asian Pac J Allergy Immunol.2013;31(1):3-10.[32] Tokcaer-Keskin Z,Akar AR,Ayaloglu-Butun F,et al.Timing of induction of cardiomyocyte differentiation for in vitro cultured mesenchymal stem cells:A perspective for emergencies.Can J Physiol Pharmacol. 2009;87(1):143-150.[33] Ronzani C,Casset A,Pons F.Exposure to multi-walled carbon nanotubes results in aggravation of airway inflammation and remodeling and in increased production of epithelium-derived innate cytokines in a mouse model of asthma.Arch Toxicol. 2014;88(2): 489-499.[34] Maarsingh H,Dekkers BG,Zuidhof AB,et al.Increased arginase activity contributes to airway remodelling in chronic allergic asthma.Eur Respir J. 2011;38(2): 318-328.[35] Bosnjak B,Tilp C,Tomsic C,et al.Tiotropium bromide inhibits relapsing allergic asthma in BALB/c mice.Pulm Pharmacol Ther.2014;27(1):44-51.[36] Hoffman SM,Tully JE,Nolin JD,et al.Endoplasmic reticulum stress mediates house dust mite-induced airway epithelial apoptosis and fibrosis.Respir Res. 2013;14(1):141.[37] Takeda N,Sumi Y,Préfontaine D,et al. Epithelium- derived chemokines induce airway smooth muscle cell migration.Clin Exp Allergy. 2009;39(7): 1018-1026.[38] Berair R,Saunders R,Brightling CE.Origins of increased airway smooth muscle mass in asthma.BMC Med.2013;11(1):145.[39] Knight DA,Rossi FM,Hackett TL.Mesenchymal stem cells for repair of the airway epithelium in asthma. Expert Rev Respir Med.2010;4(6):747-758.[40] Doyle TM,Ellis R,Park HJ,et al.Modulating progenitor accumulation attenuates lung angiogenesis in a mouse model of asthma.Eur Respir J.2011;38(3):679-687. |
.jpg)
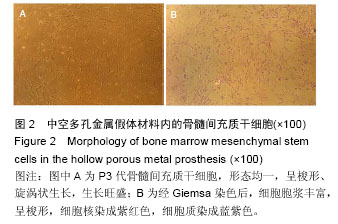
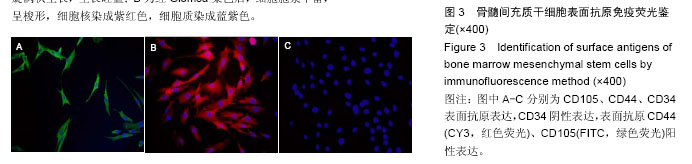
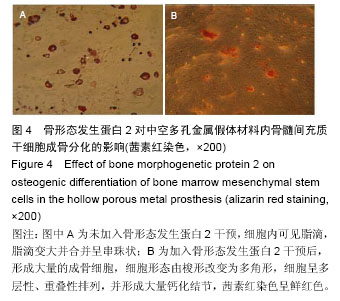
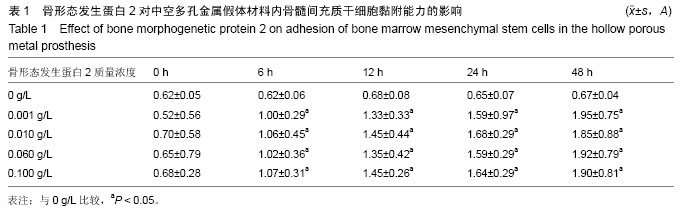
.jpg)
.jpg)
.jpg)