中国组织工程研究 ›› 2015, Vol. 19 ›› Issue (35): 5593-5597.doi: 10.3969/j.issn.2095-4344.2015.35.004
• 人工假体 artificial prosthesis • 上一篇 下一篇
唑来膦酸对髋关节置换后假体柄周围骨丢失的预防效果
刘国青1,苑振峰2,刘 鹏1,庞同涛1,张先巍1
- 1莘县人民医院骨外科,山东省莘县 252400;2泰山医学院聊城临床学院关节外科,山东省聊城市 252000
Preventive effect of zoledronic acid on bone loss around the prosthesis stem after hip replacement
Liu Guo-qing1, Yuan Zhen-feng2, Liu Peng1, Pang Tong-tao1, Zhang Xian-wei1
- 1Department of Orthopedics, People’s Hospital of Shenxian, Shenxian 252400, Shandong Province, China; 2Department of Joint Surgery, Liaocheng Clinical School Affiliated to Taishan Medical University, Liaocheng 252000, Shandong Province, China
摘要:
背景:无论是股骨侧还是髋臼侧在髋关节置换后1年内均会出现不同程度的骨丢失,对假体的长期稳定性和骨强度造成严重影响。因而减少全髋关节置换后假体柄周围骨丢失量对延长假体使用时间、预防假体周围骨折具有重要意义。
目的:观察分析唑来膦酸对髋关节置换后假体柄周围骨丢失的预防效果。
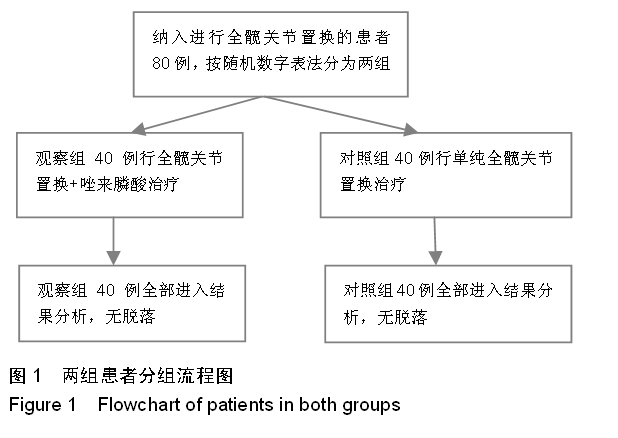
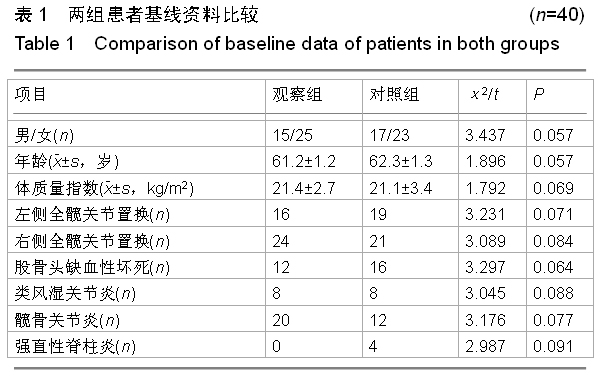
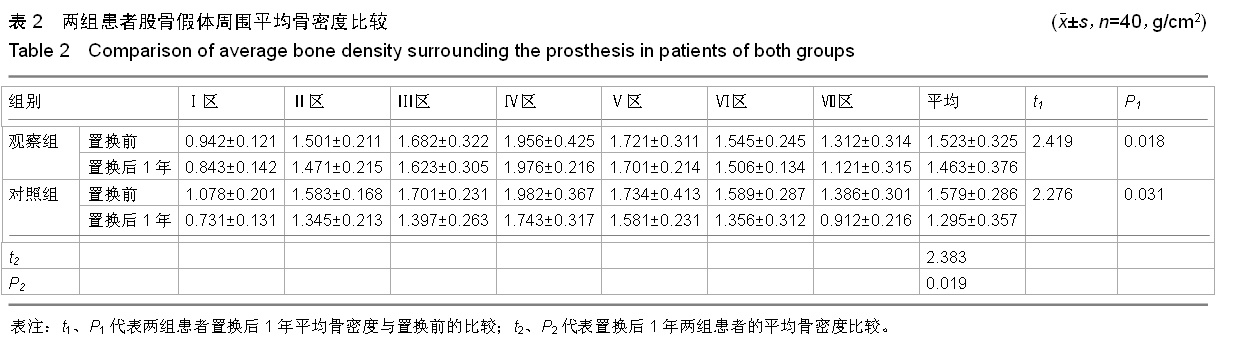
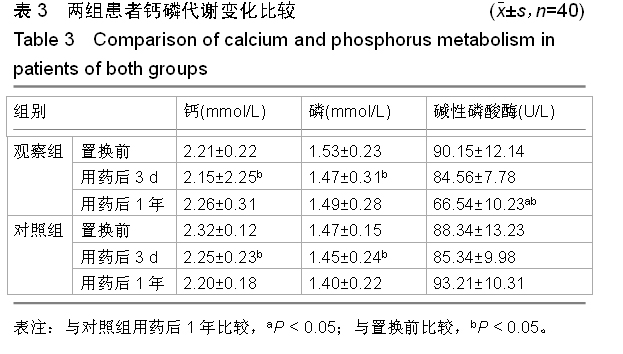
方法:对照组患者不给予,两组其余用药相同。对两组置换前和置换后1年的髋部骨密度变化进行测量;观察两组置换前、用药后3 d和用药后1年的血清钙、磷水平及碱性磷酸酶活性变化;对观察组患者用药期间出现的不良反应进行记录。
结果与结论:置换后1年两组患者股骨假体周围的平均骨密度均显著下降,与置换前比较差异有显著性意义(P < 0.05)。置换后1年观察组患者的平均骨密度显著高于对照组(P < 0.05)。用药后3 d两组患者的钙、磷水平均较置换前显著下降,差异均有显著性意义(P < 0.05)。用药后1年钙、磷水平维持在置换前水平。置换后短期内两组患者的碱性磷酸酶活性均稍微下降,但与置换前比较差异无显著性意义(P > 0.05)。用药后1年,观察组患者的碱性磷酸酶活性较低,与置换前、对照组比较差异均有显著性意义(P < 0.05)。用药后1年,对照组患者的碱性磷酸酶活性与置换前差异无显著性意义(P > 0.05)。用药两三天内观察组有9例患者出现不同程度的肌肉酸痛和发热,给予乙酰氨基酚后症状得到缓解。提示全髋关节置换后给予唑来膦酸注射液能够有效预防患者置换后早期假体柄周围骨丢失,但是置换后1周内可能会出现发热的症状,因此建议在置换完成1周后给予患者唑来膦酸注射液,若出现发热等症状,可给予乙酰氨基酚进行缓解。
中国组织工程研究杂志出版内容重点:人工关节;骨植入物;脊柱;骨折;内固定;数字化骨科;组织工程
中图分类号: