中国组织工程研究 ›› 2025, Vol. 29 ›› Issue (34): 7310-7317.doi: 10.12307/2025.492
• 纳米生物材料 nanobiomaterials • 上一篇 下一篇
铁死亡抑制剂通过活性氧途径对钴纳米颗粒毒性的抑制作用
王 琛1,张伟男2,沈冀宁2,刘 璠3,袁即山1,刘雅克3
- 1 江苏大学附属人民医院,江苏省镇江市 212000;2 南通大学,江苏省南通市 226000;3 南通大学附属医院,江苏省南通市 226000
-
收稿日期:2024-08-24接受日期:2024-09-20出版日期:2025-12-08发布日期:2025-01-17 -
通讯作者:刘雅克,博士,硕士生导师,副主任医师,南通大学附属医院,江苏省南通市 226000 -
作者简介:王琛,男,1995年生,江苏省兴化市人,汉族,江苏大学附属人民医院,住院医师,主要从事骨与关节感染及骨质疏松研究。 -
基金资助:国家自然科学基金面上项目(82172519),项目负责人:刘璠;国家自然科学基金青年科学基金项目(82002282),项目负责人:刘雅克
Inhibitory effect of ferroptosis inhibitor toxicity induced by cobalt nanoparticles through reactive oxygen species
Wang Chen1, Zhang Weinan2, Shen Jining2, Liu Fan3, Yuan Jishan1, Liu Yake3
- 1Affiliated People’s Hospital of Jiangsu University, Zhenjiang 212000, Jiangsu Province, China; 2Nantong University, Nantong 226000, Jiangsu Province, China; 3Affiliated Hospital of Nantong University, Nantong 226000, Jiangsu Province, China
-
Received:2024-08-24Accepted:2024-09-20Online:2025-12-08Published:2025-01-17 -
Contact:Liu Yake, MD, Master’s supervisor, Associate chief physician, Affiliated Hospital of Nantong University, Nantong 226000, Jiangsu Province, China -
About author:Wang Chen, Resident physician, Affiliated People’s Hospital of Jiangsu University, Zhenjiang 212000, Jiangsu Province, China -
Supported by:National Natural Science Foundation of China, No. 82172519 (to LF); National Natural Science Youth Science Foundation of China, 82002282 (to LYK)
摘要:
文题释义:
钴纳米颗粒:人工关节假体在体内长期植入后经多种因素如磨损等所释放出的纳米微粒。
铁死亡:也称为调节性细胞死亡,是一种特殊的细胞死亡形式,其特征是细胞内铁的积累和脂质活性氧的生成。
背景:目前,钴纳米颗粒引起的软组织损伤是人工关节置换患者最常见的并发症之一。因此,需要一种有效的治疗策略来限制钴纳米颗粒的毒性。
目的:探讨铁死亡抑制剂对钴纳米颗粒诱导细胞毒性的保护作用。方法:为评价铁死亡抑制剂对小鼠成纤维细胞(Balb/3T3)的解毒作用,以下实验均用钴纳米颗粒和铁死亡抑制剂处理Balb/3T3细胞24 h。细胞活力实验测定细胞存活率。根据细胞活力实验结果确定钴纳米颗粒和去铁酮浓度后,将实验分为钴纳米颗粒处理组(加入400 μmol/L钴纳米颗粒)、钴纳米颗粒+去铁酮共培养组(加入400 μmol/L钴纳米颗粒和25 μmol/L去铁酮)、去铁酮处理组(加入25 μmol/L去铁酮)及空白对照组。蛋白印迹实验检测谷胱甘肽过氧化物酶4和溶质载体家族7成员11蛋白的表达。
结果与结论:①细胞活力实验结果显示,随着作用时间延长或药物浓度的增加,细胞活力会进一步降低,表明钴纳米颗粒的细胞毒性作用是时间和剂量依赖性的。此外,暴露24 h后,与对照组相比,钴纳米颗粒引起细胞活力和谷胱甘肽水平显著降低(P < 0.05);同时,与对照组相比,活性氧生成、细胞内铁和炎症细胞因子如肿瘤坏死因子α、白细胞介素1β和白细胞介素6表达量增加。加入去铁酮后,与钴纳米颗粒组相比,细胞活力明显提高,活性氧生成、细胞内铁和炎症细胞因子如肿瘤坏死因子α、白细胞介素1β和白细胞介素6表达量也明显下降(P < 0.05)。说明去铁酮对暴露于钴纳米颗粒的细胞有明显的保护作用。②蛋白印迹实验结果显示,钴纳米颗粒降低了谷胱甘肽过氧化物酶4和溶质载体家族7成员11溶质载体家族7成员11蛋白的表达(P < 0.05),而去铁酮对此有抑制作用(P < 0.05)。③上述结果证实,钴纳米颗粒具有很强的细胞毒性,铁死亡抑制剂去铁酮对钴纳米颗粒引起的细胞毒性有解毒作用。铁死亡在钴纳米颗粒诱导细胞毒性的过程中通过活性氧途径起重要作用。铁死亡抑制剂对钴纳米颗粒毒性的抑制作用可能会有助于进一步研究钴纳米毒性作用机制及解毒方案。
https://orcid.org/0000-0002-6063-3200 (Liu Yake)
中国组织工程研究杂志出版内容重点:生物材料;骨生物材料;口腔生物材料;纳米材料;缓释材料;材料相容性;组织工程
中图分类号:
引用本文
王 琛, 张伟男, 沈冀宁, 刘 璠, 袁即山, 刘雅克. 铁死亡抑制剂通过活性氧途径对钴纳米颗粒毒性的抑制作用[J]. 中国组织工程研究, 2025, 29(34): 7310-7317.
Wang Chen, Zhang Weinan, Shen Jining, Liu Fan, Yuan Jishan, Liu Yake. Inhibitory effect of ferroptosis inhibitor toxicity induced by cobalt nanoparticles through reactive oxygen species[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7310-7317.
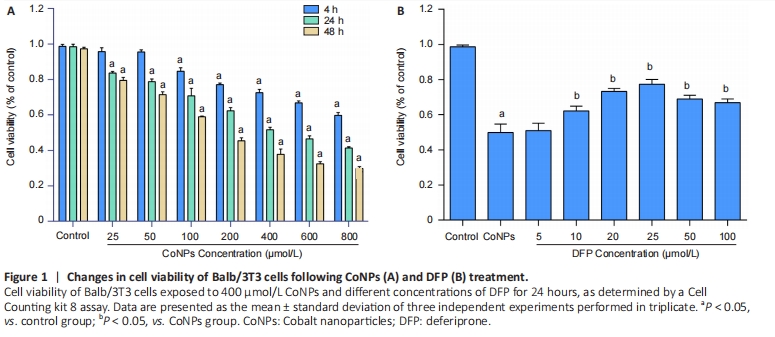
Cells were treated with different concentrations of CoNPs for 4, 24, or 48 hours and the effect of CoNPs on Balb/3T3 cell survival was determined by CCK-8 assay. The percentage of survived cells strikingly decreased with the increase of CoNPs concentration (Figure 1A). The IC50 value was 400 μmol/L 24 hours later, respectively. Cells cultured in the medium with varying concentrations of DFP showed a dose-dependent survival rate. When the concentration of DFP increased, the viability of Balb/3T3 cells increased. When cells were cultured in 400 μmol/L CoNPs and different concentrations of DFP, the viability of cells was significantly higher than the cells treated with CoNPs (Figure 1B).
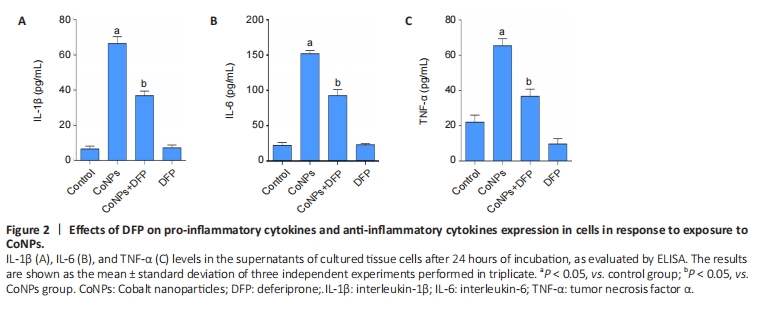
Ferroptosis inhibitor reduced inflammatory cytokines in Balb/3T3 cells
The results are shown in Figure 2. The levels of TNF-α, IL-1β, and IL-6 in the CoNPs group were noticeably higher than those in the control group. Compared with the CoNPs group, DFP treatment had a significant protective effect and significantly reduced TNF-α (P < 0.05), IL-1β (P < 0.05), and IL-6 (P < 0.05) induced by cobalt nanoparticles expression. However, there was no significant difference between the single DFP group and the CoNPs group.
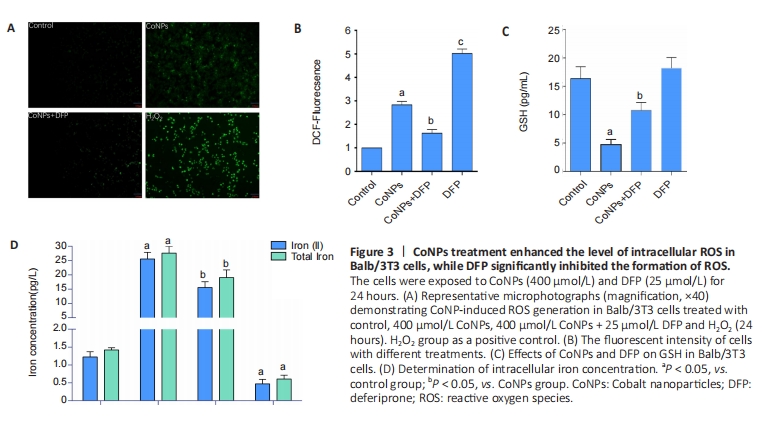
The oxidative damage induced by ROS was considered an important cause of the toxicity of cobalt nanoparticles. ROS was also the main incentive of ferroptosis. In this study, as shown in Figure 3, after 400 μmol/L CoNPs were treated for 24 hours, the ROS level of Balb/3T3 cells increased significantly (P < 0.05), while DFP (25 μmol/L) could significantly inhibit this increase (P < 0.05), and the difference was statistically significant (P < 0.05) (Figure 3A, B).
GSH is the key cellular endogenous antioxidant that can scavenge toxic free radicals, and depletion of GSH appears to promote intracellular ROS accumulation, leading to apoptosis. Altered GSH levels represent the increased cellular response to oxidative stress. The results suggested that CoNPs treatment decreased GSH levels (P < 0.05), and DFP inhibited this decrease (P < 0.05) as measured by the GSH assay kit (Figure 3C).
ROS promotes lipid peroxidation, leading to damage to the cell membrane and eventual cell death. When the concentration of iron ions in the cell increases, the likelihood of ferroptosis is significantly heightened. Conversely, reducing the intracellular free iron content or using iron chelators can effectively inhibit or slow down the process of ferroptosis[25-26]. Our results showed that CoNPs could lead to significant increases in Fe2+ ion and total iron concentration in Balb/3T3 cells compared with the control group. However, the increase was inhibited when cells were exposed to DFP (Figure 3D). These results indicate that CoNPs can induce the occurrence of ferroptosis in Balb/3T3 cells by stimulating oxidative stress.
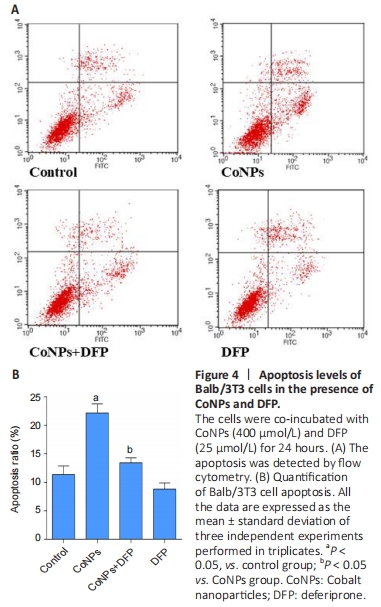
Apoptosis assay in Balb/3T3 cells
To investigate the apoptosis rate of cells treated with 400 μmol/L CoNPs and 25 μmol/L DFP, the AnnexinV-FITC assay kit was used. Incubation with 400 μmol/L CoNPs for 24 hours mildly increased the cell apoptosis rate compared with the control group. When DFP was added as a treatment before exposure to CoNPs, the apoptosis was obviously alleviated (Figure 4A, B).
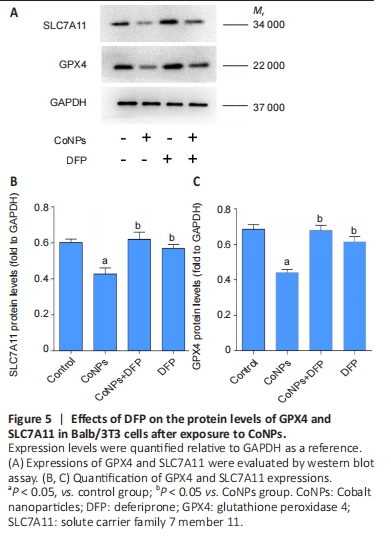
Ferroptosis inhibitor exerted the positive regulation on GPX4 in Balb/3T3 cells
GPX4 is the central regulator of ferroptosis, and the decline of GPX4 is often used as a marker of ferroptosis[27-28]. SLC7A11 is a unit of the glutamate-cystine antiporter Xc-, resulting in enhanced lipid oxidation and ferroptosis. Western blot assay showed that CoNPs noticeably decreased the protein amount of GPX4 and SLC7A11. However, the decrease in GPX4 and SLC7A11 levels was inhibited when cells were treated with 25 μmol/L DFP and 400 μmol/L CoNPs for 24 hours. These results suggest that CoNPs might induce ferroptosis and the ferroptosis inhibitor DFP exerts therapeutic action against CoNPs (Figure 5).
| [1] SINGH JA, SLOAN JA. Health-related quality of life in total hip and total knee arthroplasty. Rheumatology (Oxford). 2008;47(12):1826-1831. [2] JACOBS J, HALLAB N, SKIPOR A, et al. Metal degradation products: a cause for concern in metal-metal bearings? Clin Orthop Relat Res. 2003;(417):139-147. [3] LIN X, CHEN C, CHEN J, et al. Long Noncoding RNA NR_030777 alleviates cobalt nanoparticles-induced neurodegenerative damage by promoting autophagosome-lysosome fusion. ACS Nano. 2024; 18(36):24872-24897. [4] LIU Y, ZHU W, NI D, et al. Alpha lipoic acid antagonizes cytotoxicity of cobalt nanoparticles by inhibiting ferroptosis-like cell death. J Nanobiotechnology. 2020;18(1):141. [5] SABBIONI E, FORTANER S, FARINA M, et al. Cytotoxicity and morphological transforming potential of cobalt nanoparticles, microparticles and ions in Balb/3T3 mouse fibroblasts: an in vitro model. Nanotoxicology. 2014;8(4):455-464. [6] PAPAGEORGIOU I, BROWN C, SCHINS R, et al. The effect of nano- and micron-sized particles of cobalt-chromium alloy on human fibroblasts in vitro. Biomaterials. 2007;28(19):2946-2958. [7] WAN R, MO Y, ZHANG Z, et al. Cobalt nanoparticles induce lung injury, DNA damage and mutations in mice. Part Fibre Toxicol. 2017;14(1):38. [8] MONTEILLER C, TRAN L, MACNEE W, et al. The pro-inflammatory effects of low-toxicity low-solubility particles, nanoparticles and fine particles, on epithelial cells in vitro: the role of surface area. Occup Environ Med. 2007;64(9):609-615. [9] COLOGNATO R, BONELLI A, PONTI J, et al. Comparative genotoxicity of cobalt nanoparticles and ions on human peripheral leukocytes in vitro. Mutagenesis. 2008;23(5):377-382. [10] RAJIV S, JEROBIN J, SARANYA V, et al. Comparative cytotoxicity and genotoxicity of cobalt (II, III) oxide, iron (III) oxide, silicon dioxide, and aluminum oxide nanoparticles on human lymphocytes in vitro. Hum Exp Toxicol. 2016;35(2):170-183. [11] HOREV-AZARIA L, KIRKPATRICK CJ, KORENSTEIN R, et al. Predictive toxicology of cobalt nanoparticles and ions: comparative in vitro study of different cellular models using methods of knowledge discovery from data. Toxicol Sci. 2011;122(2):489-501. [12] Martin O, VLADIMIR G, STEN O, et al. Mitochondria, oxidative stress and cell death. Apoptosis. 2007;12(5):913-922. [13] MASUI T, SAKANO S, HASEGAWA Y, et al. Expression of inflammatory cytokines, RANKL and OPG induced by titanium, cobalt-chromium and polyethylene particles. Biomaterials. 2005;26(14):1695-1702. [14] GOODMAN SB, HUIE P, SONG Y, et al. Cellular profile and cytokine production at prosthetic interfaces. Study of tissues retrieved from revised hip and knee replacements. J Bone Joint Surg Br. 1998;80(3):531-539. [15] DIXON SJ, LEMBERG KM, LAMPRECHT MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060-1072. [16] HAN C, LIU Y, DAI R, et al. Ferroptosis and its potential role in human diseases. Front Pharmacol. 2020;11:239. [17] XIE Y, HOU W, SONG X, et al. Ferroptosis: process and function. 2016; 23(3):369-379. [18] YANG WS, SRIRAMARATNAM R, WELSCH ME, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156(1-2):317-331. [19] JIANG L, KON N, LI T, et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520(7545):57-62. [20] ZOU Y, SCHREIBER SL. Progress in understanding ferroptosis and challenges in its targeting for therapeutic benefit. Cell Chem Biol. 2020;27(4):463-471. [21] IMAI H, MATSUOKA M, KUMAGAI T, et al. Lipid peroxidation-dependent cell death regulated by GPx4 and ferroptosis. Curr Top Microbiol Immunol. 2017;403:143-170. [22] ZHANG Y, SUN C, ZHAO C, et al. Ferroptosis inhibitor SRS 16-86 attenuates ferroptosis and promotes functional recovery in contusion spinal cord injury. Brain Res. 2019;1706:48-57. [23] LIU Y, HONG H, LU X, et al. L-ascorbic acid protected against extrinsic and intrinsic apoptosis induced by cobalt nanoparticles through ROS attenuation. Biol Trace Elem Res. 2017;175(2):428-439. [24] JIANG H, LIU F, YANG H, et al. Effects of cobalt nanoparticles on human T cells in vitro. Biol Trace Elem Res. 2012;146(1):23-29. [25] LI J, CAO F, YIN HL, et al. Ferroptosis: past, present and future. Cell Death Dis. 2020;11(2):88. [26] PANG Q, TANG Z, LUO L. The crosstalk between oncogenic signaling and ferroptosis in cancer. Crit Rev Oncol Hematol. 2024;197:104349. [27] YANG W, SRIRAMARATNAM R, WELSCH M, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156(1-2):317-331. [28] FRIEDMANN ANGELI J, SCHNEIDER M, PRONETH B, et al. Inactivation of the ferroptosis regulator GPX4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16(12):1180-1191. [29] WANG S, WANG C, ZHANG W, et al. Bioactive nano-selenium antagonizes cobalt nanoparticle-mediated oxidative stress via the Keap1-Nrf2-ARE signaling pathway. J Nanopart Res. 2022;24(1):1-12. [30] SABBIONI E, FORTANER S, FARINA M, et al. Cytotoxicity and morphological transforming potential of cobalt nanoparticles, microparticles and ions in Balb/3T3 mouse fibroblasts: an in vitro model. Nanotoxicology. 2014;8(4):455-464. [31] YAN X, LIU Y, XIE T, et al. alpha-Tocopherol protected against cobalt nanoparticles and cocl2 induced cytotoxicity and inflammation in Balb/3T3 cells. Immunopharmacol Immunotoxicol. 2018;40(2):179-185. [32] LU LQ, TIAN J, LUO XJ, et al. Targeting the pathways of regulated necrosis: a potential strategy for alleviation of cardio-cerebrovascular injury. Cell Mol Life Sci. 2021;78(1):63-78. [33] THOMAS V, HALLORAN B, AMBALAVANAN N, et al. In vitro studies on the effect of particle size on macrophage responses to nanodiamond wear debris. Acta Biomater. 2012;8(5):1939-1947. [34] HALLAB NJS. Biologic responses to orthopedic implants: innate and adaptive immune responses to implant debris. Spine. 2016;41 Suppl 7:S30-S31. [35] KAUFMAN A, ALABRE C, RUBASH H, et al. Human macrophage response to UHMWPE, TiAlV, CoCr, and alumina particles: analysis of multiple cytokines using protein arrays. J Biomed Mater Res A. 2008;84(2):464-474. [36] DIXON SJ, PRATT DA. Ferroptosis: a flexible constellation of related biochemical mechanisms. Mol Cell. 2023;83(7):1030-1042. [37] IMAI H, MATSUOKA M, KUMAGAI T, et al. Lipid peroxidation-dependent cell death regulated by GPx4 and ferroptosis. Curr Top Microbiol Immunol. 2017;403:143-170. [38] CHEN D, FAN Z, RAUH M, et al. ATF4 promotes angiogenesis and neuronal cell death and confers ferroptosis in a xCT-dependent manner. Oncogene. 2017;36(40):5593-5608. [39] ZHANG W, WANG C, ZHU W, et al. Ferrostatin-1 alleviates cytotoxicity of cobalt nanoparticles by inhibiting ferroptosis. Bioengineered. 2022; 13(3):6163-6172. [40] ZHU W, ZHANG R, LIU S, et al. The effect of nanoparticles of cobalt-chromium on human aortic endothelial cells in vitro. J Appl Toxicol. 2021;41(12):1966-1979. |
| [1] | 杨学涛, 朱梦菡, 张宸熙, 孙一民, 叶 玲. 抗氧化纳米材料在口腔中的应用和不足[J]. 中国组织工程研究, 2026, 30(8): 2044-2053. |
| [2] | 陈豪杰, 王 黛, 沈 山. 种植体周围炎中的免疫炎症微环境机制[J]. 中国组织工程研究, 2026, 30(8): 2054-2062. |
| [3] | 刘大为, 崔颖颖, 王方辉, 王子轩, 陈宇涵, 李友瑞, 张荣和. 表没食子儿茶素没食子酸酯介导活性氧双向调控及在纳米材料中的应用[J]. 中国组织工程研究, 2026, 30(8): 2101-2112. |
| [4] | 刘宏杰, 牟秋菊, 申玉雪, 梁 飞, 祝丽丽. 金属有机框架/羧甲基壳聚糖-氧化海藻酸钠/富血小板血浆水凝胶促糖尿病感染创面愈合[J]. 中国组织工程研究, 2026, 30(8): 1929-1939. |
| [5] | 王菘芃, 刘玉三, 于焕英, 高晓丽, 徐英江, 张晓明, 刘 敏. 沸石基咪唑盐框架8纳米材料的活性氧双向调控:从肿瘤治疗、抗菌到细胞保护[J]. 中国组织工程研究, 2026, 30(8): 2033-2013. |
| [6] | 彭志伟, 陈 雷, 佟 磊. 木犀草素促进糖尿病小鼠创面愈合的作用与机制[J]. 中国组织工程研究, 2026, 30(6): 1398-1406. |
| [7] | 朱奎成, 杜春燕, 章金涛. 无毛基因突变促进无毛小鼠白色脂肪组织褐变的作用机制[J]. 中国组织工程研究, 2026, 30(6): 1424-1430. |
| [8] | 李 豪, 陶红成, 曾 平, 刘金富, 丁 强, 牛驰程, 黄 凯, 康宏誉. 丝裂原活化蛋白激酶信号通路调控骨关节炎的发生发展:指导中药靶点治疗[J]. 中国组织工程研究, 2026, 30(6): 1476-1485. |
| [9] | 陈 驹, 郑锦畅, 梁 振, 黄成硕, 林 颢, 曾 莉. β-石竹烯对小鼠膝骨关节炎的作用及机制[J]. 中国组织工程研究, 2026, 30(6): 1341-1347. |
| [10] | 温广伟, 甄颖豪, 郑泰铿, 周淑怡, 莫国业, 周腾鹏, 李海山, 赖以毅. 异银杏素对破骨细胞分化的影响和机制[J]. 中国组织工程研究, 2026, 30(6): 1348-1358. |
| [11] | 吕国庆, 艾孜麦提江·肉孜, 熊道海. 鸢尾素抑制人关节软骨细胞中铁死亡的作用及其机制[J]. 中国组织工程研究, 2026, 30(6): 1359-1367. |
| [12] | 彭团辉, 宋洪明, 杨 玲, 丁小歌, 蒙鹏骏. 长期耐力运动对自然衰老小鼠kl/FGF23轴及钙磷代谢的影响[J]. 中国组织工程研究, 2026, 30(5): 1089-1095. |
| [13] | 阴勇成, 赵相瑞, 杨志杰, 李 政, 李 芳, 宁 斌. 过氧化物还原酶1在脊髓损伤后小胶质细胞炎症反应中的作用及机制[J]. 中国组织工程研究, 2026, 30(5): 1106-1113. |
| [14] | 胡 静, 朱 伶, 谢 娟, 孔德营, 刘豆豆. 自噬影响组蛋白修饰标记H3K4me3调控小鼠早期胚胎发育[J]. 中国组织工程研究, 2026, 30(5): 1147-1155. |
| [15] | 陈伊娴, 陈 晨, 卢立恒, 汤锦鹏, 于晓巍. 雷公藤甲素治疗骨关节炎的网络药理学分析与实验验证[J]. 中国组织工程研究, 2026, 30(4): 805-815. |
The term ferroptosis was proposed by Dixon et al. in 2012 to describe the form of cell death induced by small molecules such as erastin[15]. Ferroptosis is dependent upon intracellular iron, accumulation of lipid ROS, and loss of activity of the lipid repair enzyme glutathione peroxidase 4 (GPX4). Ferroptosis is a recently recognized form of regulated cell death involving iron accumulation and lipid peroxidation, and its death phenotype is different from other types of cell death (such as apoptosis and necrosis)[15]. The depletion of GPX4, the down-regulation of SLC7A11 activity, and the increase of lipid peroxidation products are common features of ferroptosis[16-17]. At the same time, GPX4 is also an important regulator of ferroptosis[18]. The expression of SLC7A11 can potentially improve the survival rate of cells by regulating iron metabolism[19]. In addition, SLC7A11 is an essential component of the amino acid antiporter system Xc- and plays a crucial protective role in GSH synthesis. Since its recognition, ferroptosis has been involved in degenerative diseases such as the kidney and brain and plays an important role in a variety of cancers[20]. The accumulation of reactive oxygen species plays a key role in causing ferroptosis[21], which is also the main trigger for the toxicity of CoNPs. In addition, ferroptosis inhibitors have been shown to have anti-inflammatory effects by inhibiting the expression of inflammatory cytokines, including TNF-α[22]. The inflammatory response is also one of the toxic factors of CoNPs. Therefore, we speculate that ferroptosis inhibitors can reduce the toxicity of CoNPs based on the inhibition of inflammation and ROS.
In a challenge to previous findings, we explored the iron chelator deferiprone (DFP) as a ferroptosis inhibitor to antagonize the toxicity of cobalt nanoparticles by depletion of ROS. This study presents a novel approach to mitigating the cytotoxic effects of cobalt nanoparticles, which are a significant concern for patients with artificial joint prostheses. The innovation lies in the exploration of the iron chelator as a therapeutic strategy against CoNP-induced toxicity. Specifically, the study demonstrates that deferiprone provides significant protection against CoNP-induced cytotoxicity in Balb/3T3 cells. 中国组织工程研究杂志出版内容重点:生物材料;骨生物材料;口腔生物材料;纳米材料;缓释材料;材料相容性;组织工程
In vitro cytological basic experiment, using one-way analysis of variance.
Time and settings
The experiment was completed in the Clinical Research Center of Affiliated Hospital of Nantong University and the Central Laboratory of Affiliated People’s Hospital of Jiangsu University from January 2022 to January 2024.
Materials
CoNPs (30 nm, 99.9%) were produced by Shanghai Chaowei Nano Technology Company. Dulbecco’s modified Eagle’s medium, 2’,7’-dichlorofluorescein diacetate (DCFH-DA), fetal bovine serum, trypsin-eDtA and penicillin/streptomycin were purchased from Gibco (Life Technologies, Paisley, UK). Tissue culture dishes were obtained from Corning Inc. (New York, USA). Cell counting kit-8 (CCK-8) kit and ROS detection kit were purchased from Beyotime Company (Jiangsu, China). Mouse TNF-α, IL-1β, and IL-6 enzyme-linked immunosorbent assay (ELISA) kits were purchased from R&D Systems (Minneapolis, MN, USA). Annexin V-FITC was purchased from Multi Sciences (Hangzhou, China). Iron assay kit and all antibodies were purchased from Abcam (Cambridge, UK). 10% polyacrylamide gel premix (NCM FastPAGE) was purchased from New Cell & Molecular Biotech Co., Ltd. (Suzhou, China). Deferiprone was gotten from MCE (New Jersey, USA).
Methods
Preparation of cobalt nanoparticles
The methods of the CoNP characterization were described in our previous work[23]. The size, microstructure, and elemental composition of CoNPs were assessed by X-ray diffraction, high-resolution scanning electron microscopy and transmission electron microscopy. CoNP samples were sterilized at 180℃ for 4 hours and suspended in ultrapure water to achieve the initial concentration of 40 mmol/L. Freshly prepared stock solution was ultrasonicated at room temperature at 40 W for 30 minutes to minimize particle aggregates and diluted to achieve the required concentrations (25, 50, 100, 200, 400, and 800 µmol/L) with a complete culture medium.
Preparation of DFP
DFP (1 mmol/L) was irradiated and sterilized under ultraviolet light for 2 hours and then diluted to 25 µmol/L with basal medium for subsequent experiments. The processed DFP was stored at −20°C.
Cell culture and sample treatment
The Balb/3T3 cell line was bought from the cell repository of the Chinese Academy of Sciences. Briefly, cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, penicillin, and streptomycin. The medium was replaced every 3 days. All cells were grown at 37 ℃ in a humidified incubator containing 5% CO2. For the dose-related experiments, the cells were exposed to CoNPs for 4, 24, and 48 hours. To examine the protective effects of DFP on the viability of cells, the cells were treated with varying concentrations of DFP and exposed to a target concentration of CoNPs for 24 hours.
Cell viability assay
According to the manufacturer’s protocol, Balb/3T3 cells were incubated in 96-well plates and then treated with different concentrations of DFP and 400 μmol/L CoNPs for 4, 24, or 48 hours. After that, 10 μL CCK-8 was added to each well and incubated at 37 ℃ for another 4 hours. The absorbance was measured by the microplate reader at 450 nm. Cell viability ratio was expressed as percent control. From this, the 50 % inhibitory concentration (IC50 value) was calculated[24].
Detection of cytokines
Cells were plated at a density of 1×105 cells/well in six-well plates and incubated for 24 hours at 37 ℃. Then the cells were exposed to CoNPs (400 µmol/L) with or without the treatment of DFP (25 µmol/L) for 24 hours. After treatment, cytokines levels of the supernatants collected from cell cultures were measured. The concentrations of TNF-α, IL-1β, and IL-6 in the culture media were determined using ELISA kits according to the manufacturer’s instructions. Each sample was analyzed in triplicate.
ROS measurement
Spectrofluorometry analysis and fluorescence microscopy imaging were used to assess ROS generation in cells exposed to target concentrations of CoNPs and the protective effects of DFP treatment. For the spectrofluorometry analysis, cells (1×105/well) were seeded in 96-well plates. Balb/3T3 cells were exposed to CoNPs and ferroptosis inhibitor for 24 hours. Following completion of the DCFH-DA reaction, the reaction mixture was washed thrice with 200 mL of PBS in each well. The fluorescence intensity of the suspension was measured on a multi-well microplate reader using excitation and emission of 485 nm and 525 nm, respectively. Values were expressed as the percentage of fluorescence intensity relative to the control wells.
Detection of intracellular iron ion concentration
Balb/3T3 cells were seeded into a 6-well tissue culture plate. When the cells were in the logarithmic growth phase, the cells were treated with 25 µmol/L DFP and exposed to
400 μmol/L CoNPs for 24 hours. The cells were collected, washed with 1 mL PBS, and centrifuged at 1 000 r/min for 5 minutes to remove the supernatant. After that, samples were treated with 500 μL of lysis buffer and placed on a shaker to fully lyse for 2 hours, then centrifuged at 12 000 r/min for 20 minutes to collect supernatant. According to the manufacturer’s instructions, the intracellular iron ion concentration and total iron were detected using the Iron Assay Kit by spectrophotometry.
GSH measurement
The cells were treated with 25 µmol/L DFP and exposed to 400 μmol/L CoNPs for 24 hours. After the exposure, the cells were washed with PBS twice, scraped off, suspended in PBS, and centrifuged at 1 000 × g. The cell pellet was then homogenized in 5% 5-sulfosalicylic acid. Next, the suspension was lysed by freezing and thawing twice for 5 minutes and centrifuged at 10,000 × g for 10 minutes. The amount of GSH was measured according to the manufacturer’s protocol.
Quantitative assessment of cell apoptosis detected by flow cytometry
For the measurement of cell apoptosis, the AnnexinV-FITC assay kit was conducted according to the manufacturer’s protocol. Briefly, cells were washed with PBS solution and harvested. Next, the cells were suspended in a binding buffer at 1×106 cells/mL. A 100 mL cell suspension was withdrawn, supplemented with 5 mL AnnexinV-FITC and 10 mL of PI, and subsequently incubated for 15 minutes at room temperature in the dark. A Becton Dickinson flow cytometer was employed for the measurement within 1 hour of staining.
Western blot assay
The cells were treated with 25 µmol/L DFP and exposed to target concentrations of CoNPs for 24 hours. Cells were washed in cold PBS and lysed in RIPA with 1 mmol/L phenyl sulfonyl fluoride (Beyotime, Nantong, China). The supernatants were harvested and protein concentrations were measured with the bicinchoninic acid assay kit. The concentrated samples were subjected to 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride filter membrane by a transfer apparatus at 300 mA for 1 hour. The membranes were blocked for 1 hour and then incubated with primary antibodies [rabbit monoclonal to anti-GPX4 antibody (1:1 000, #ab125066, Abcam), rabbit polyclonal to Anti-xCT antibody (1:1 000, #ab37185, Abcam), and mouse monoclonal to anti-GAPDH antibody (1:1 000, #ab59164, Abcam)] at 4 ℃ overnight. After washing with TBST, the membranes were incubated with anti-mouse IgG secondary antibody (1:5 000, #L3202, SAB) for 2 hours at room temperature. The polyvinylidene difluoride filter membranes were washed four times with TBST according to the manufacturer’s protocol. We analyzed the resulting bands, which represented the target protein, based on their molecular weight.
Main observations
Screening for the safe concentration range of CoNPs on cells and the detoxifying concentration of DFP. Detecting the inhibition of CoNP-induced inflammatory cytokine expression in cells by DFP. Investigating the impact of DFP intervention on CoNP-induced ROS production in cells. Assessing the effect of DFP intervention on the concentration of iron ions in cells. Examining the influence of DFP intervention on GSH production in cells. Evaluating the impact of DFP intervention on cell apoptosis. Detecting the effects of DFP intervention on the expression of GPX4 and SLC7A11 in cells.
Statistical analysis
All statistical analyses were performed using GraphPad Prism software 7 (GraphPad Software Inc., La Jolla, CA, USA) using one-way analysis of variance followed by Dunnett’s test to evaluate significance relative to control. Results summarized data from three independent experiments and were represented as the mean ± standard deviation. Differences between groups were considered significant if P-values < 0.05.
Our results suggest that DFP treatment can reduce the decline in cell viability induced by CoNPs. The cell viability of the experimental group treated with the ferroptosis inhibitor was much higher than that of the control group, which indicated that the ferroptosis inhibitor had a certain protective effect on Balb/3T3 cells.
Studies have shown that cobalt-chromium wear particles can induce the release of a variety of pro-inflammatory cytokines, such as TNF-α, IL-1β, and IL-6[33-35]. In this study, we confirm the pro-inflammatory effect of CoNPs through the increase of TNF-α, IL-1β, and IL-6 and suggest that ferroptosis inhibitors have a protective effect on inflammatory cytokines in Balb/3T3 cells induced by 400 μmol/L CoNPs.
CoNPs induced a noticeable increase in ROS. ROS is considered the main cause of ferroptosis, which can cause DNA damage, and oxidative stress and activate other cellular processes. On the one hand, ROS acts as a dynamic balance signal molecule that regulates cell growth, and on the other hand, it acts as an adaptive response at physiological concentrations. However, the production of ROS at higher concentrations can cause cell damage and death. GSH is an important antioxidant substance that plays an important role in protecting against apoptosis. The depletion of GSH promotes ROS accumulation in cells and cell apoptosis. Our results show that CoNPs treatment decreased GSH levels and DFP reversed this decrease as measured by the GSH assay kit. Moreover, DFP could significantly suppress CoNP-induced increment of intracellular Fe2+ ion and total iron concentration in Balb/3T3 cells.
Flow cytometry results exhibited that CoNPs induced cellular apoptosis, which could be further alleviated by the exposure of DFP. Although the apoptosis rate of Balb/3T3 cells for the CoNPs group was significantly increased compared to the control group, it was still much lower than that of the control group, as shown by CCK-8 under the same conditions (Figure 1). These results indicate that non-apoptotic mechanisms are involved in CoNP-mediated cell death, which is different from past opinions[36].
GPX4 and SLC7A11 are recognized as important endogenous regulators of ferroptosis, and the decline of GPX4 and SLC7A11 is always regarded as a marker of ferroptosis[37-38]. A previous study has indicated that SLC7A11 protects cells from ferroptosis by increasing GSH production[39]. In this study, our results strongly support the idea that ferroptosis inhibitors can upregulate the expression of GPX4 and SLC7A11 to inhibit the cytotoxicity caused by CoNPs.
There are some limitations in this study. Firstly, CoNPs induce cell death through various complex signaling pathways, including ferroptosis, apoptosis, necrosis, autophagy, and pyroptosis. DFP participates in ferroptosis by regulating iron metabolism, lipid peroxidation, and glutathione levels[40]. Additionally, CoNPs affect cell survival through other mechanisms. Therefore, comprehensive consideration of multiple signaling pathways is necessary when studying their toxicity mechanisms. In addition, while the study provides valuable insights, further research, including animal models, is necessary to fully understand the implications in vivo.
In summary, all these results strongly supported the idea that the ferroptosis inhibitor counteracts cytotoxicity and inflammation induced by CoNPs. Ferroptosis plays an important role in the occurrence of CoNP-induced toxicity. In addition, we are supposed to compare the results of different ferroptosis inhibitors on cell detoxification and further screen out the most suitable drugs to relieve the CoNP-induced toxicity produced in artificial joints. The research provides a new perspective for antagonizing CoNP-induced cytotoxicity. Future studies should focus on exploring the underlying molecular mechanisms in greater detail and validating these findings in in vivo models. 中国组织工程研究杂志出版内容重点:生物材料;骨生物材料;口腔生物材料;纳米材料;缓释材料;材料相容性;组织工程
 #br#
#br#
文题释义:#br# 钴纳米颗粒:人工关节假体在体内长期植入后经多种因素如磨损等所释放出的纳米微粒。#br# 铁死亡:也称为调节性细胞死亡,是一种特殊的细胞死亡形式,其特征是细胞内铁的积累和脂质活性氧的生成。
钴纳米颗粒引起的软组织损伤是人工关节置换患者最常见的并发症之一。因此,需要一种有效的治疗策略来限制钴纳米颗粒的毒性。文章旨在探讨铁死亡抑制剂对钴纳米颗粒诱导细胞毒性的保护作用。
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||